SUSTech Chuang-Chuang Li’s group publishes review on natural products containing highly strained trans-fused bicyclo[3.3.0]octane
Wen ZHANG and Lingzi LI 2021-08-12
Recently, Professor Chuang-Chaung Li’s group from the Department of Chemistry at the Southern University of Science and Technology (SUSTech) published a review article in the academic journal Chemical Society Reviews, entitled “Synthesis of natural products containing highly strained trans-fused bicyclo[3.3.0]octane: historical overview and future prospects.” It is the first review article in this field. It aims to offer a helpful reference for the total synthesis of highly strained natural products containing trans-fused bicyclo[3.3.0]octane ring systems.
Strained natural products are beneficial for their pharmacological capabilities and binding to the desired biological targets tightly and selectively. They are unique and fascinating gifts from nature, providing promising bioactivities against cancer and other diseases, such as Taxol, a famous anticancer drug.
Notably, highly strained trans-fused bicyclo[3.3.0]octane was reported that trans-fused bicyclo[3.3.0]octane is more unfavorable (by ∼13.0 and ∼6.4 kcal/mol of strain energy [SE]) than its cis-fused counterpart by calculation and experiment, respectively. Therefore, molecules with trans-fused bicyclo[3.3.0]octane ring systems are very difficult to synthesize, and there are very few approaches to access them as the development of methodology is slow.
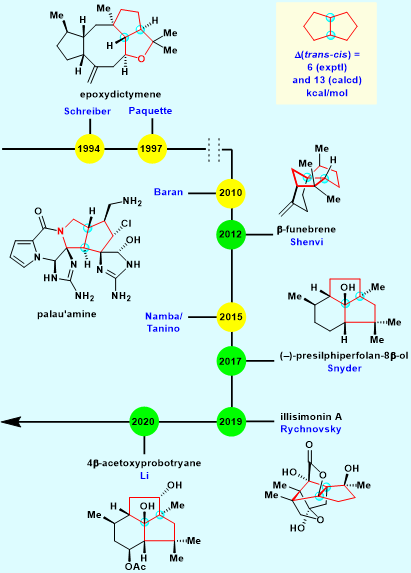 Figure 1. Synthetic progress of natural products containing trans-fused bicyclo[3.3.0]octane
Figure 1. Synthetic progress of natural products containing trans-fused bicyclo[3.3.0]octane
The total synthesis of this high strain structure stands as a significant synthetic challenge for decades. Great chemists from the synthetic community such as Prof. Schreiber (Harvard), Baran (Scripps), Shenvi (Scripps), Namba/Tanino (Hokkaido University), Snyder (University of Chicago), Rychnovsky (UCSD), and Chuang-Chuang Li (SUSTech) have made significant progress in this field recently. Many new strategies have been applied in total synthesis successfully.
In 2020, Chuang-Chuang Li’s group first utilized a unique benzilic acid type rearrangement under very mild conditions, synthesizing highly strained trans-fused bicyclo[3.3.0]octane cleverly. The first and asymmetric total synthesis of 4β-acetoxyprobotryane-9β,15α-diol was then completed. This work provided new access to the synthesis of highly strained trans-fused bicyclo[3.3.0]octane and lay the foundation for further biological research (J. Am. Chem. Soc., 2020, 142, 19868).
In this review, the total syntheses of natural products containing trans-fused bicyclo[3.3.0]octane have been described from a historical viewpoint covering the literature before 2021. A systematic and comprehensive discussion on the synthesis is also provided. Prospects in this field are presented at the end of the paper and are a valuable reference for its future development.
Dr. Wen Zhang and Dr. Lingzi Li, both from the Department of Chemistry at SUSTech, are the first and co-authors, respectively. Prof. Chuang-Chuang Li from the Department of Chemistry at SUSTech is the sole corresponding author.
This work was supported by the National Natural Science Foundation of China (NSFC), Guangdong Provincial Key Laboratory of Catalysis, Research Projects of Universities of Guangdong Province, and the Shenzhen Peacock Plan.
Paper link: https://doi.org/10.1039/D0CS01471K
Previous JACS paper link: https://doi.org/10.1021/jacs.0c10116




