Researchers make progress in discovering oral anti-SARS-CoV-2 drug
2022-05-20
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus driving the ongoing coronavirus disease 2019 (COVID-19) pandemic, continues to rapidly evolve. Due to the limited efficacy of vaccination in the prevention of SARS-CoV-2 transmission and the continuous emergence of variants of concern (VOC), orally bioavailable and broadly efficacious antiviral drugs are urgently needed.
Professor Xumu Zhang, Vice Dean of the College of Science at the Southern University of Science and Technology (SUSTech) and Head of the Medi-X Pingshan, and Professor Deyin Guo, Dean of the School of Medicine at Sun Yat-Sen University (SYSU), launched an intensive collaboration to identify an effective and accessible treatment against COVID-19.
Their recent progression in the study of an oral antiviral drug, entitled “The adenosine analog prodrug ATV006 is orally bioavailable and has preclinical efficacy against parental SARS-CoV-2 and variants”, was published in the Science Translational Medicine, a leading journal in the field of translational research at the intersection of science and medicine.
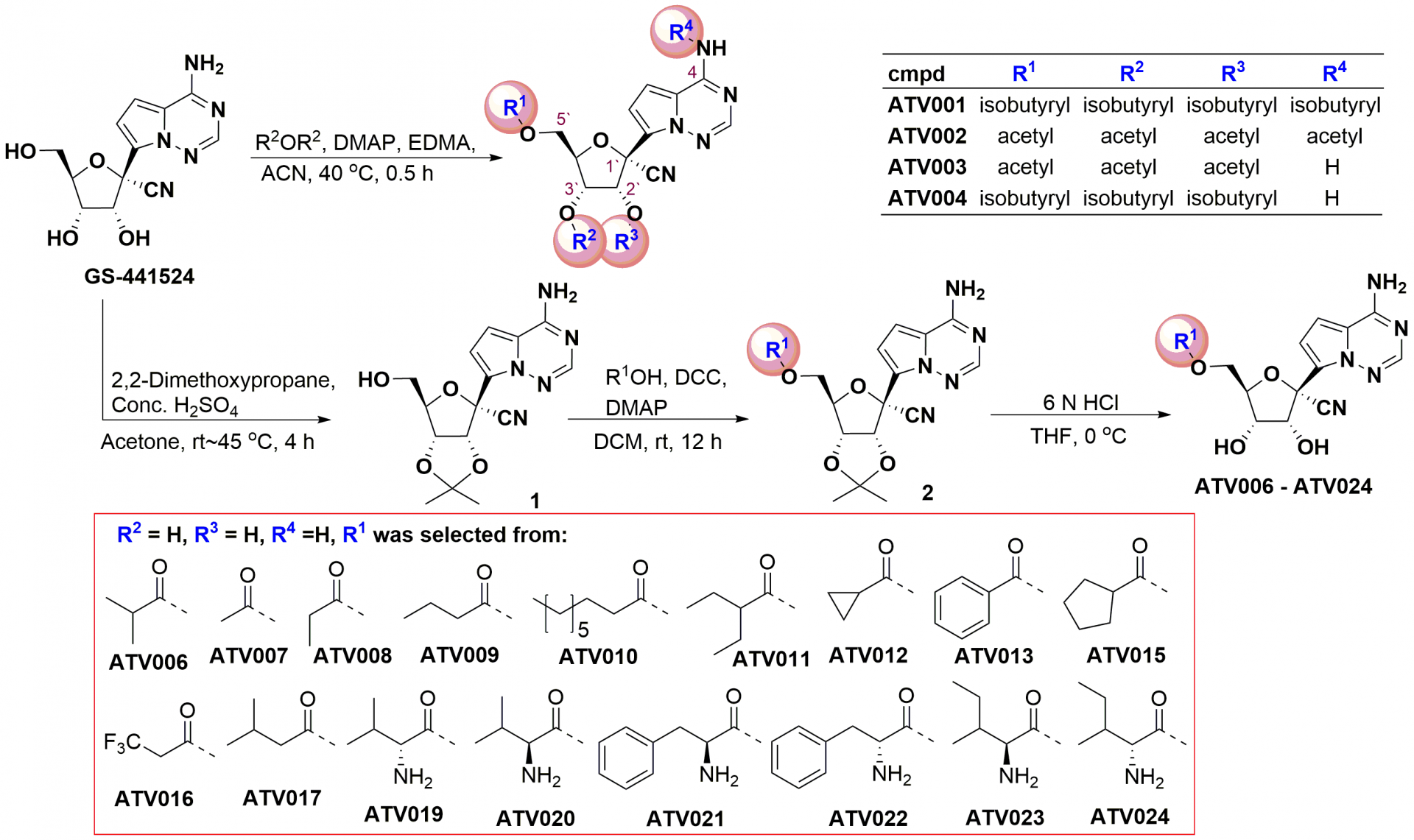
Figure 1. The in vitro evaluation of GS-441524-derived prodrugs
Previously, they reported that GS-441524, the major metabolite and the parent nucleotide of remdesivir, has a better inhibitory activity on SARS-CoV-2 and mouse hepatitis virus (MHV)-A59. However, the unfavorable oral PK prevented its further development into an oral drug. In the current article, they reported that the 5′-hydroxyl-isobutyryl prodrug, ATV006, demonstrated excellent oral bioavailability in rats and cynomolgus monkeys and exhibited potent antiviral efficacy against different SARS-CoV-2 VOCs in vitro and in three mouse models.
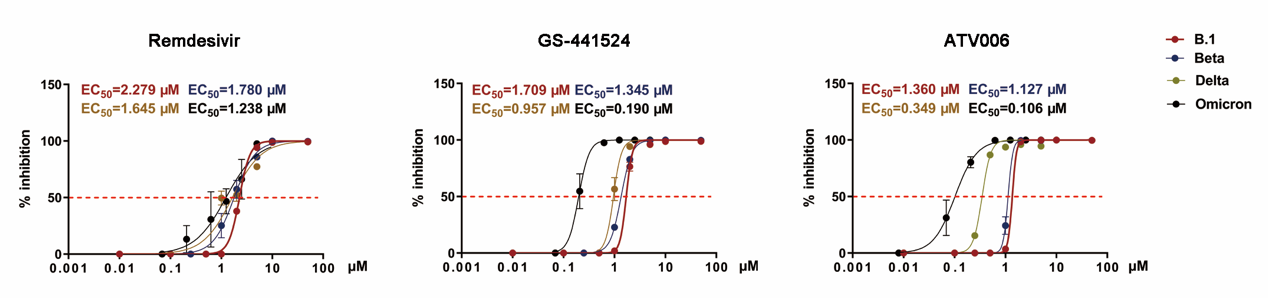
Figure 2. ATV006, remdisivir, and GS-441524 exhibit antiviral activity against parental SARS-CoV-2 and variants in vitro
Oral ATV006 displayed high oral bioavailability (F%) of 81.5 % and Cmax of 8.2 µM in Sprague Dawley rats, following dosing of 25 mg/kg. The activated forms of ATV006, GS-441524, monophosphate, and triphosphate achieved broad distribution in the plasma, liver, and kidneys as well as in the lung, the major target organ for SARS-CoV-2 infection. The EC50 values of ATV006 against Delta and Omicron variants were 0.349 and 0.106 µM, respectively. Oral administration of ATV006 reduced viral loads and alleviated lung damage when administered prophylactically and therapeutically to K18-hACE2 mice challenged with the Delta variant of SARS-CoV-2.
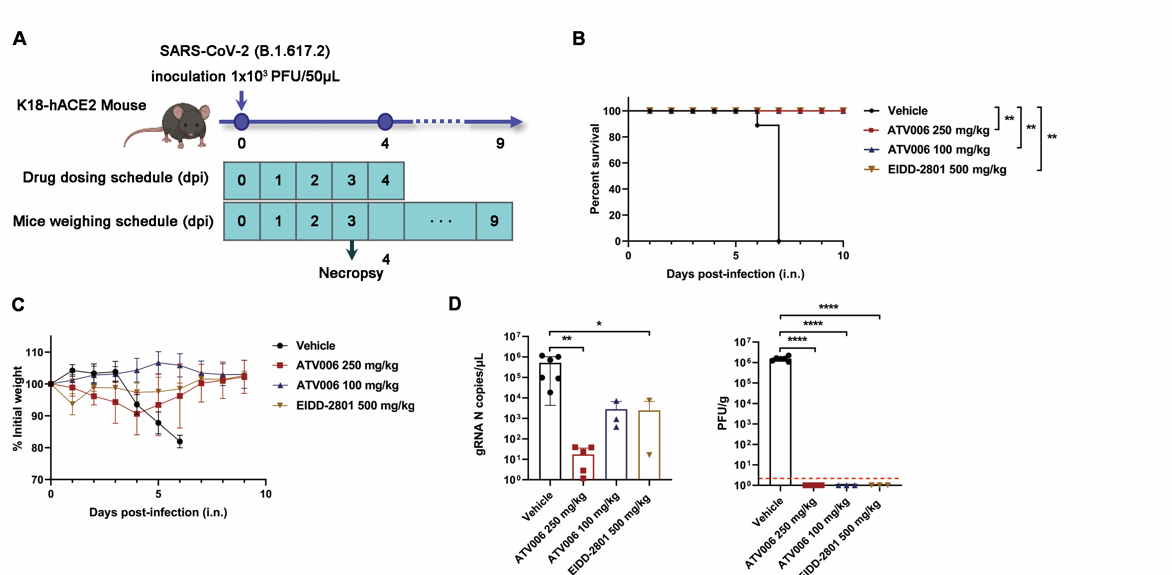
Figure 3. Oral ATV006 treatment reduces viral load, prevents lung pathology, and prevented the death of mice infected with SARS-CoV-2
Compared to remdesivir, ATV006 is structurally simpler and easier to synthesize via a three-step transformation with GS-441524 as starting material, which would reduce the cost and accelerate mass production. Conversely, remdesivir with complex chiral phosphoramide prodrug is difficult and costly to synthesize. In addition, ATV006 has good water solubility and oral druggability. This data suggest that ATV006 represents a promising and simplified nucleoside prodrug strategy for the treatment of COVID-19 and potentially for emerging coronavirus diseases in the future. Further extensive evaluation of other prodrugs in the same series are ongoing.
This project was a multidisciplinary collaboration by researchers from Guangdong Province. Yingjun Li and Qifan Zhou from SUSTech, Liu Cao and Sidi Yang from SYSU, Guanguan Li from the Medi-X Pingshan, and Jing Sun from Guangzhou Medical University (GMU) are the co-first authors of this paper. Prof. Xumu Zhang and Yingjun Li from SUSTech, Prof. Deyin Guo from SYSU, and Prof. Jincun Zhao from GMU are the co-corresponding authors.
Researchers from the Second Affiliated Hospital of SUSTech (Shenzhen Third People’s Hospital), Guangzhou Customs District Technology Center (GZESL), Guangdong Laboratory Animals Monitoring Institute, and the Guangdong Provincial Center for Disease Control and Prevention also contributed to this work.
This project was supported by the Shenzhen Science and Technology Innovation Commission, the National Natural Science Foundation of China (NSFC), the National Key Research and Development Program of China, as well as the Guangdong Province Key Area Research and Development Program. This work represents a precise and comprehensive drug research at SUSTech.
Paper link:




