Patrick J. Walsh shares his research at Science Lecture
2024-04-29
On April 25, 2024, Prof. Patrick J. Walsh from the University of Pennsylvania was invited to the 147 Science Lecture in the College of Science. He gave a lecture themed “C-C and C-N Bond Formation through 1- and 2-Electron Processes”, which was chaired by Associate Professor Tiezheng JIA of the Department of Chemistry, SUSTech.
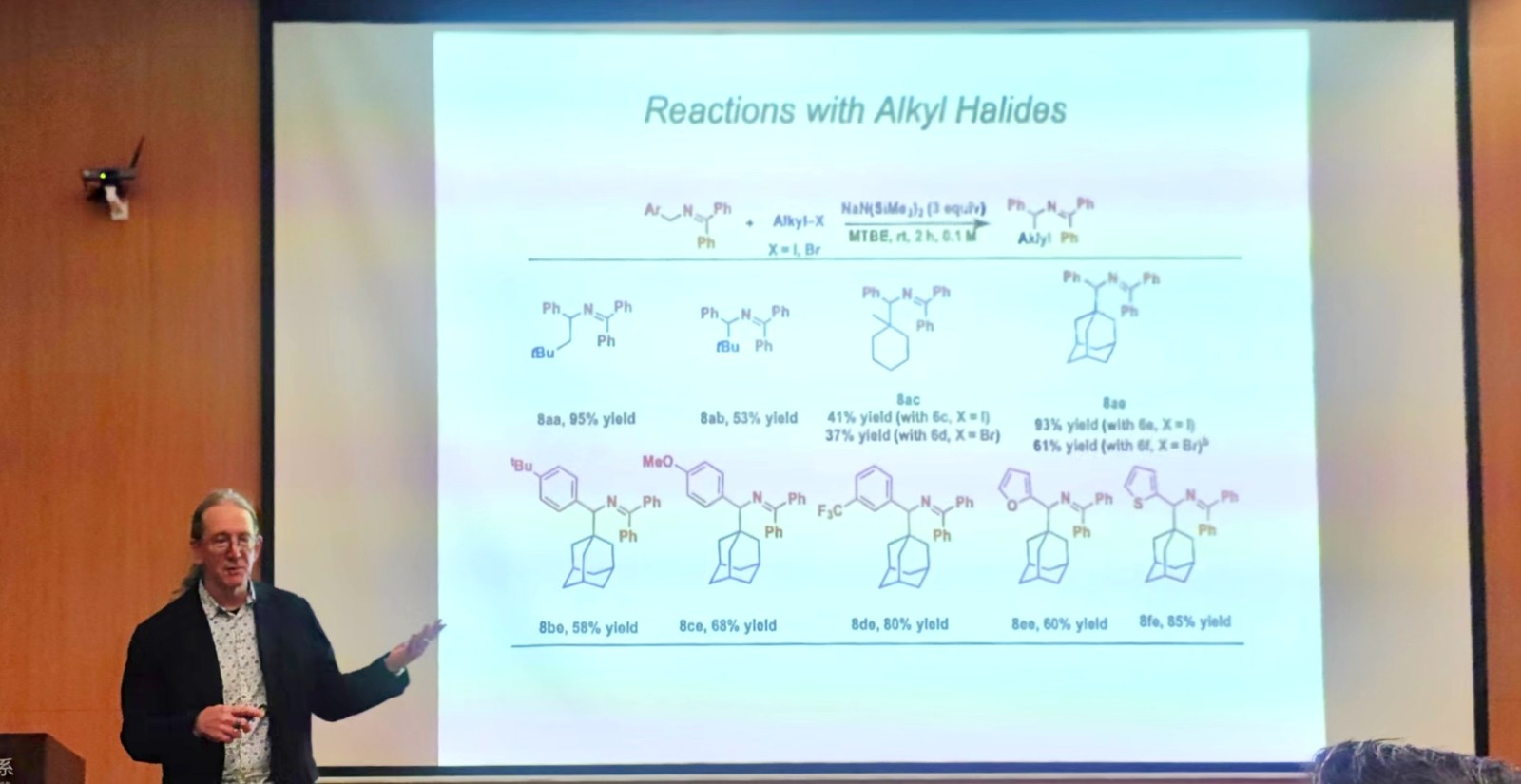
In the lecture, Prof. Patrick J. Walsh introduced that his team has been interested in the fascinating chemistry of 2-azaallyl anions, which involves both SET reactions and two-electron processes. They have found that 2-azaallyl anion undergoes cross-coupling with aryl halides and hindered alkyl iodides via SET reactions followed by radical-radical coupling in the absence of transition metal catalysts. This mode of reactivity has been used to prepare valuable heterocycles. A wide range of chemistries has been discovered, including unusual 1,2-HAT reactions and enantioselective metal-catalyzed processes with 2-azaallyl radical intermediates derived from 2-azadienes.
Subsequently, he introduced the use of sulfenate anion as organocatalysts. He developed an organocatalytic approach to synthesize alkynes from benzyl chlorides and benzaldehyde derivatives. He shared an example of the coupling of benzyl halides using sulfenate anion as organocatalysts to generate trans-stilbenes. The method also could be used for benzylic chloromethyl coupling polymerization. He has developed a sulfenate anion-catalyzed method to synthesize trans-aziridines from imines and benzylic or alkyl halides with high yields and diastereoselectivities.
In the Q&A interactive session, the on-site teachers and students on the spot actively asked questions about the reactivity of 2-azaallyl anions, the application scenarios of subsequent synthesis and the activity of sulfenate anion organocatalysts. Prof. Patrick J. Walsh provides detailed answers one by one.
Q: Using negative ions to produce free radicals, this species is very active, so how to control the cross-coupling of free radicals, and inhibit the homocoupling of free radicals?
A: This can be explained through persistent free radical effects. The reaction system produces two kinds of free radicals: one is transient free radical and the other is persistent free radical. Transient free radicals can react with persistent free radicals or homocoupling occurs, so a small number of homocoupling byproducts are produced in the reaction. With the consumption of transient free radicals, the concentration of persistent free radicals will increase, so as long as the initial stage of the reaction is passed, the concentration of persistent free radicals in the reaction system will be far greater than that of transient free radicals, thus making it easier for transient free radicals to react with persistent free radicals.
Q: Does 2-azaallyl anions directly reduce alkyl halide substrates?
A: Yes, they have the ability to directly reduce alkyl halide substrates, mainly through a single electron transfer process (SET) between anions and alkyl halide substrates. Compared with transition metal reducing agents, 2-azaallyl anions have the advantages of lower costs and toxicities.
In conclusion, Chair Professor Xumu ZHANG and Associate Professor Tiezheng JIA handed an honorary certificate to Prof. Patrick J. Walsh.





