Researchers unveil unusual ambiphilicity through isolation of crystalline stannyne
2024-06-20
Most transition metal complexes feature partially occupied d orbitals, rendering their valence orbitals capable of both nucleophilic and electrophilic interactions with small molecules. In stark contrast, main group elements typically possess fully occupied valence electrons. The chemical reactivity of main group compounds is closely tied to the energy levels of frontier molecular orbitals and the distribution of their valence electrons. This reactivity is further influenced by factors such as atomic radius and electronegativity.
However, recent studies have revealed that main group compounds in low oxidation states with coordinative unsaturation exhibit frontier orbital characteristics and chemical behavior akin to those of transition metals. These compounds, displaying “transition-metal-like” behavior, are predominantly characterized by their ambiphilicity. Such ambiphilic molecules not only activate inert small molecules but also hold significant promise in catalysis and materials science.

Associate Professor Liu Leo Liu and his team from the Department of Chemistry at the Southern University of Science and Technology (SUSTech) have advanced the field of ambiphilic main group chemistry, focusing on main group element multiple bonds that exhibit both nucleophilic and electrophilic.
Their groundbreaking research, published in Nature Chemistry under the title “A Crystalline Stannyne”, demonstrates the synthesis of a stannyne molecule (R1−C≡Sn−R2 ↔ R1−C(:)−Sn(:)−R2) through strategic π-donation effects and steric protection from substituents.
This work has not only expanded our understanding of main group elements by featuring diverse adjacent ambiphilic centers, but also highlighted the importance of novel main group structures in synthetic chemistry, medicinal chemistry, materials science, and other related fields.
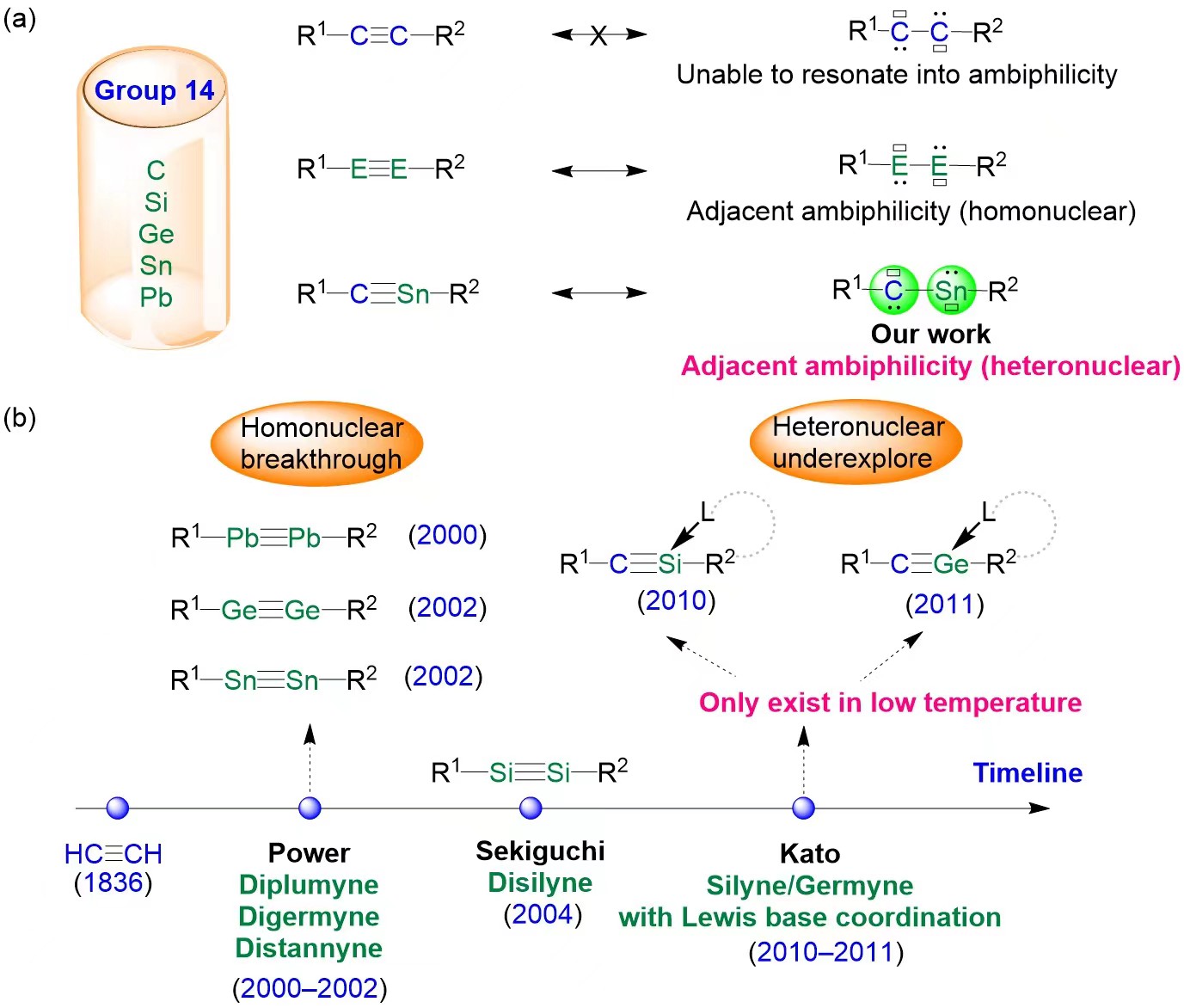
Figure 1. (a) Alkynes and their group 14 heavier element analogs; (b) The historical development of group 14 heavier element analogs of alkynes; L stands for a Lewis base.
While the alkyne molecule (R1−C≡C−R2) cannot resonate into two ambiphilic carbene centers (Figure 1a), its heavier analogs show fundamentally different electronic structures. Due to the inertness of the lone pairs of electrons on heavy element centers and the reluctance of heavier elements to engage in s/p hybridization, which weakens the formation of multiple bonds, heavier alkyne analogs display adjacent ambiphilic properties (R1−E≡E−R2 ↔ R1−E(:)−E(:)−R2; E = Si, Ge, Sn, Pb).
It is worth noting that since the discovery of acetylene in 1836, heteronuclear alkynes that incorporate a heavier group 14 element (R1−C≡E−R2 ↔ R1−C(:)−E(:)−R2; E = Si, Ge, Sn, Pb) have been a challenging target, eluding synthesis and characterization (Figure 1).
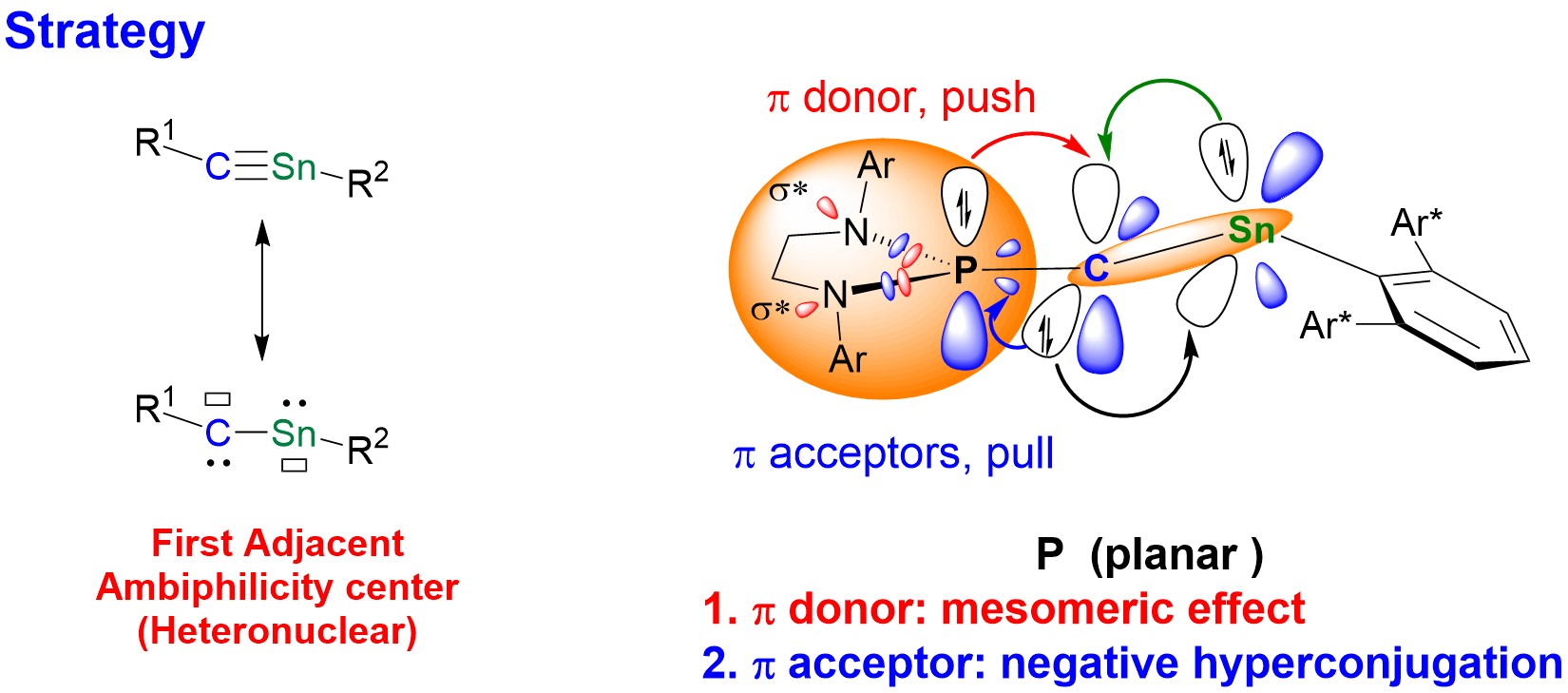
Figure 2. The resonance structures of stannyne and the strategy for stabilizing stannyne.
The research group stabilized an ambiphilic stannyne (R1−C≡Sn−R2 ↔ R1−C(:)−Sn(:)−R2) using a π-electron “push-pull” strategy alongside bulky substituents (Figure 2). This achievement represents the first instance of constructing adjacent ambiphilic centers of heteronuclear elements (Figure 3a), addressing a significant challenge in the field of main group chemistry.
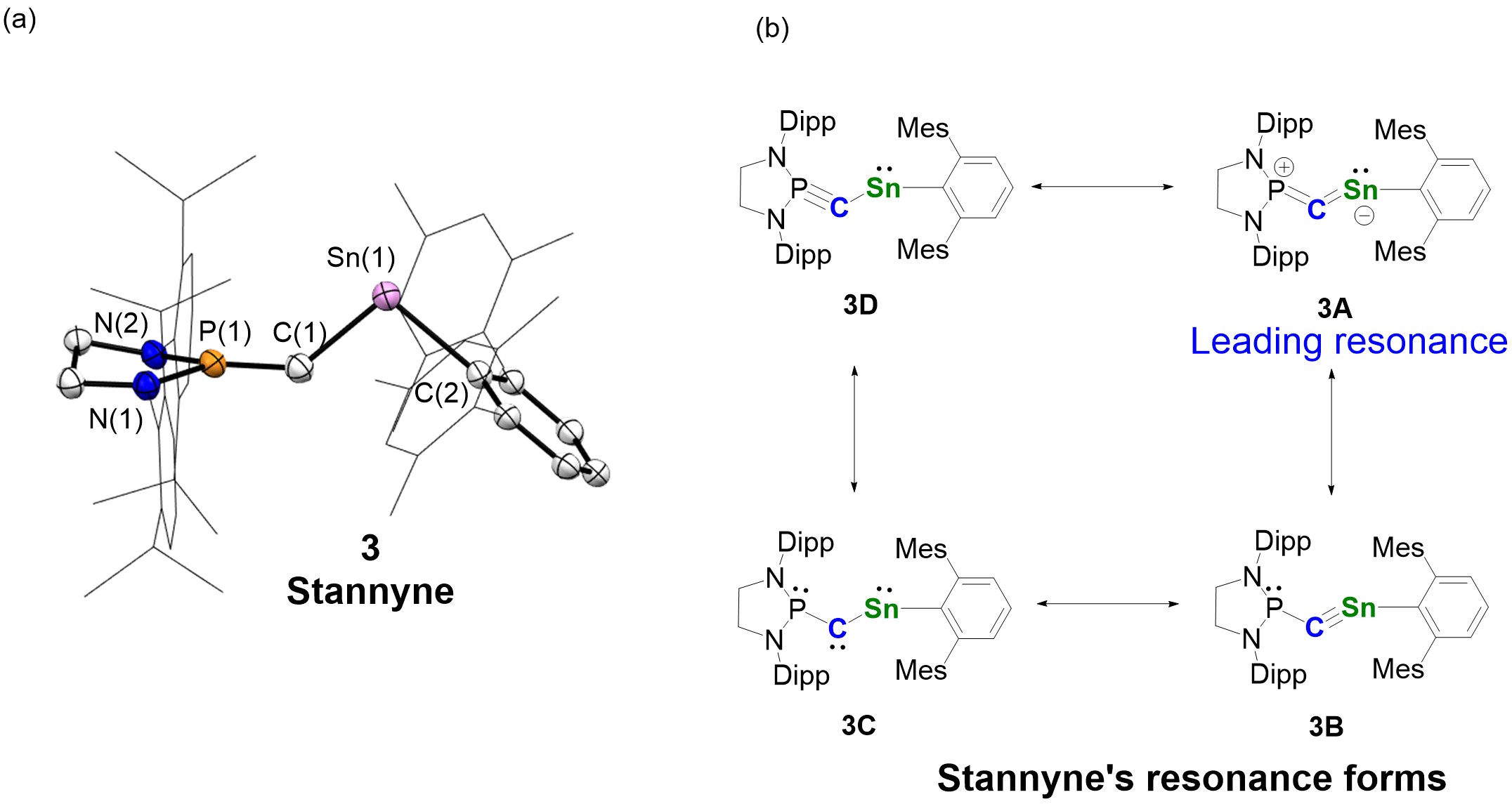
Figure 3. The solid structure and resonance forms of stannyne (Image source: Nature Chemistry).
Nuclear magnetic resonance and theoretical calculations confirm that stannyne 3 exists as a singlet species. The leading resonance form of stannyne is the allenic structure 3A (Figure 3b). Theoretical calculations revealed that the carbon atom in stannyne behaves as an ambiphilic carbene with a reverse electronic configuration (σ0π2 type). Through experimental studies, the researchers explored the reactivity of stannyne. All compounds were characterized using nuclear magnetic resonance, crystallography, and high-resolution mass spectrometry, among other methods (Figure 4).
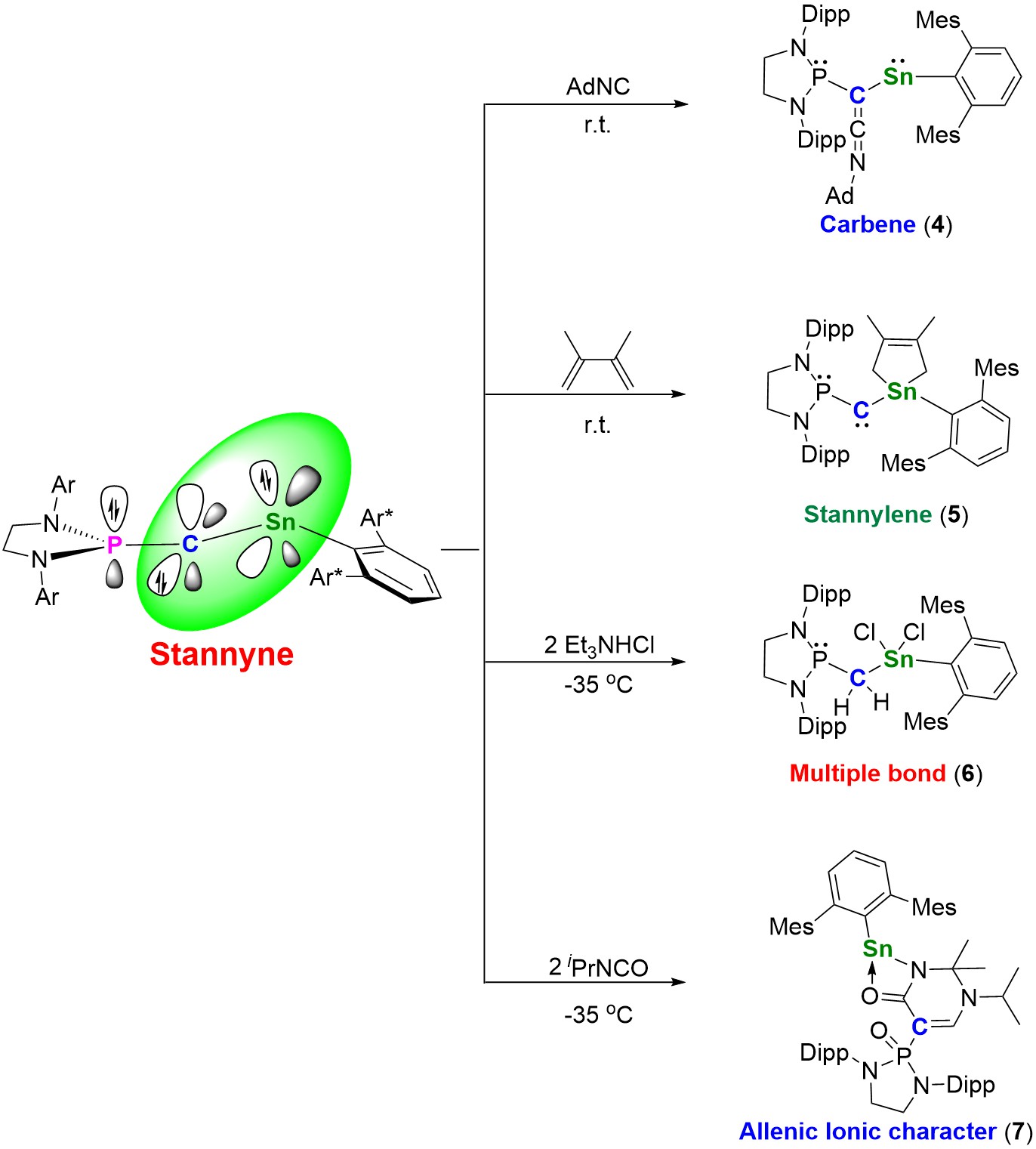
Figure 4. Reactivity of Stannyne 3; (Image Source: Nature Chemistry).
This study represents a significant advancement in the field of heavier alkyne analogs, enriching our comprehension of heavy-element chemistry. Owing to their distinctive electronic properties, stannyne compounds possess the potential to fundamentally transform the development of ambiphilic main group compounds, heralding new possibilities in the synthesis and application of these materials.
The research group led by Associate Professor Liu Leo Liu has been dedicated to the study of ambiphilic main group chemistry. With a commitment to ground-breaking innovations within main group frameworks, they have tackled the core scientific challenge of stabilizing (multi-active) ambiphilic main group compounds. Through thoughtful design and synthesis, they have successfully isolated, characterized, and generated in-situ a variety of novel ambiphilic compounds, profoundly investigating their chemical properties and potential applications.
To date, the group’s significant contributions have been recognized in leading scientific journals, including Science, Nature Chemistry, Nature Synthesis, Chem, CCS Chemistry, Journal of the American Chemical Society, and Angewandte Chemie International Edition. Furthermore, their research has been featured several times as highlights or previews by ChemistryViews, Nature Synthesis, Chem, and Synfacts.
Xin-Feng Wang, a Ph.D. student at SUSTech, is the first author of the paper. Associate Professor Liu Leo Liu is the corresponding author, and SUSTech is the sole affiliated unit. Other contributors to this work included group members Dr. Chaopeng Hu, Dr. Jiancheng Li, Dr. Rui Wei, and Dr. Xin Zhang.
This research was supported by the National Natural Science Foundation of China (NSFC), Shenzhen Science and Technology Innovation Program, Guangdong Innovation & Entrepreneurial Research Team Program, High Level of Special Funds, and the Guangdong Provincial Key Laboratory of Catalysis.
Paper link: https://www.nature.com/articles/s41557-024-01555-4




