Researchers use chemically modified electrodes to enable electrocatalytic cyclic deracemization
2024-08-01
Deracemization stands as a promising, novel method for constructing chiral centers by selectively converting racemic mixtures into single enantiomers. Despite significant progress in thermochemical and photocatalytic deracemization, electrochemical deracemization has remained a persistent challenge, with no previous reports until recently. In a 2023 review, Professor Sanzhong Luo from Tsinghua University commented, “… electrode, magnetic field, etc., have a chance to expand the boundary of deracemization”.
The biggest challenge in achieving deracemization reactions lies in breaking the microscopic reversibility in kinetics. Traditionally, the compatibility issue between oxidants and reductants has been a significant obstacle.
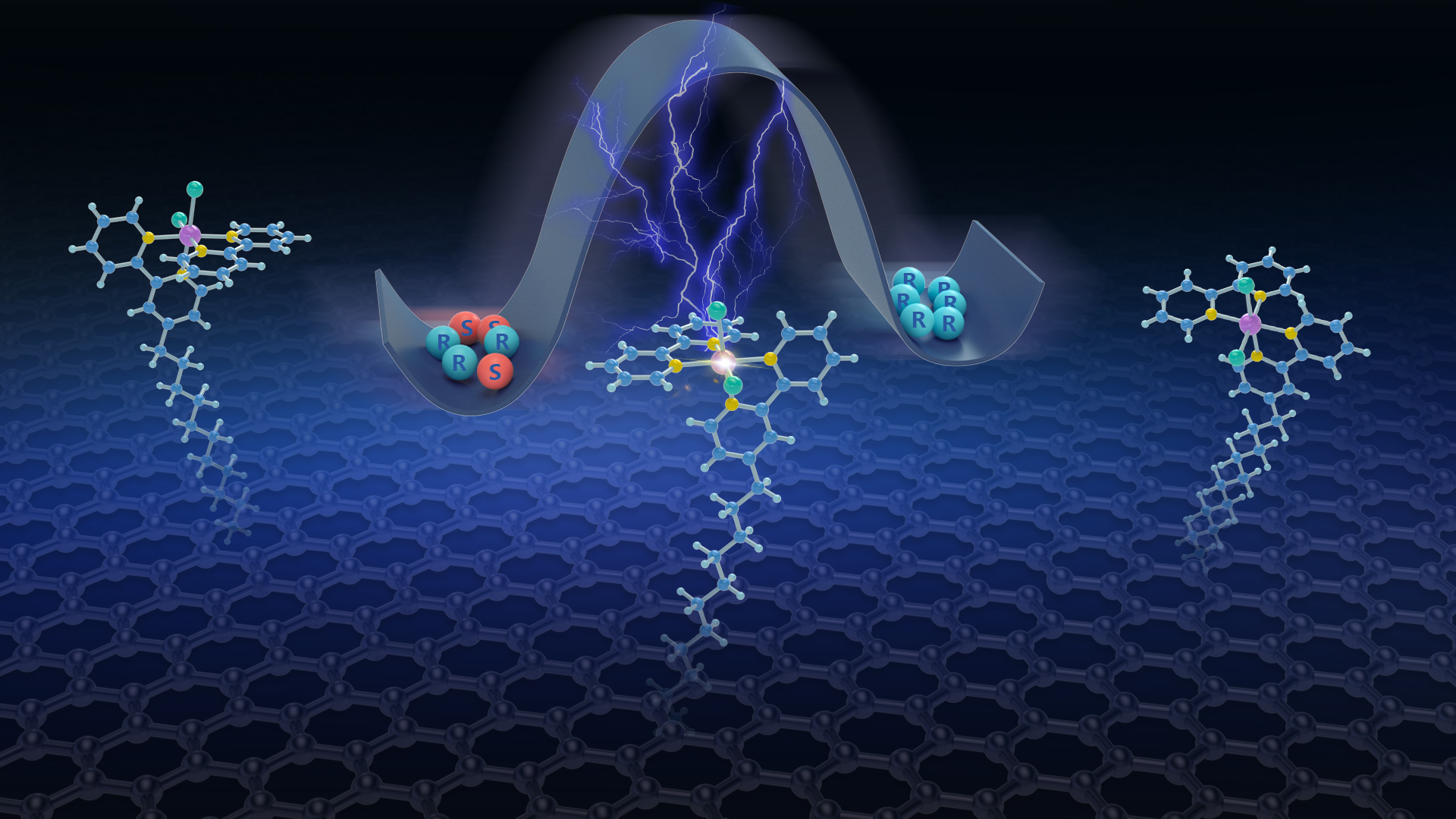
A research team led by Associate Professor Jianchun Wang from the Department of Chemistry and the Shenzhen Grubbs Institute at the Southern University of Science and Technology (SUSTech) has successfully utilized a novel chemically modified electrode strategy to overcome the compatibility issue of redox potentials for different catalysts. Their achievement includes the electrochemical deracemization of secondary alcohols.
Their work, entitled “Electrocatalytic Cyclic Deracemization Enabled by a Chemically Modified Electrode”, has been published in Nature Catalysis.
The researchers proposed utilizing metal-hydride catalysis, combined with anodic oxidation kinetic resolution and cathodic reduction, to achieve the so-called cyclic deracemization. The specific design idea is that at the anode, one configuration of the substrate is selectively oxidized to a ketone through the chiral catalyst M1 , while M1−H is reoxidized to M1 (Figure 1). At the cathode, the ketone is reduced back to a racemic pair of alcohols. Even if the resolution selectivity is not high, multiple cycles can gradually increase the enantiomeric excess value.
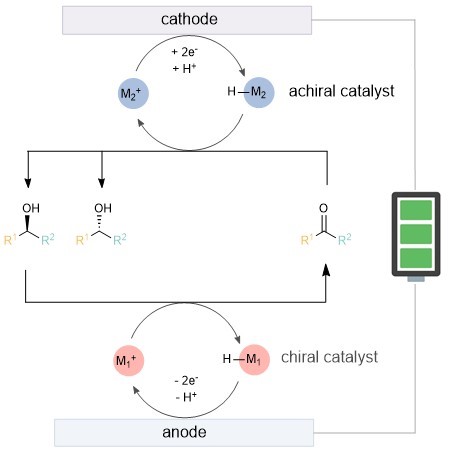
Figure 1. Preliminary reaction design
However, the issue of redox compatibility between the two formally different metal-hydride species still exists. The team turned their attention to chemically modified electrodes (CMEs), which are rarely involved in organic synthesis. By using an electrode modified with a rhodium complex as the cathode and a Noyori-type catalyst as the catalyst for anodic oxidation, they successfully achieved the electrochemical deracemization of secondary alcohols (Figure 2).
Using the modified electrode strategy, the rhodium complex was fixed on the surface of the cathode, preventing unnecessary oxidative side reactions and making the rhodium complex more easily reducible. This overcame the redox incompatibility of the two catalytic species. It is worth noting that this reaction can only be achieved using the chemically modified electrode Rh-CME. If a homogeneous rhodium complex Rh-3 is used, this process cannot be realized.
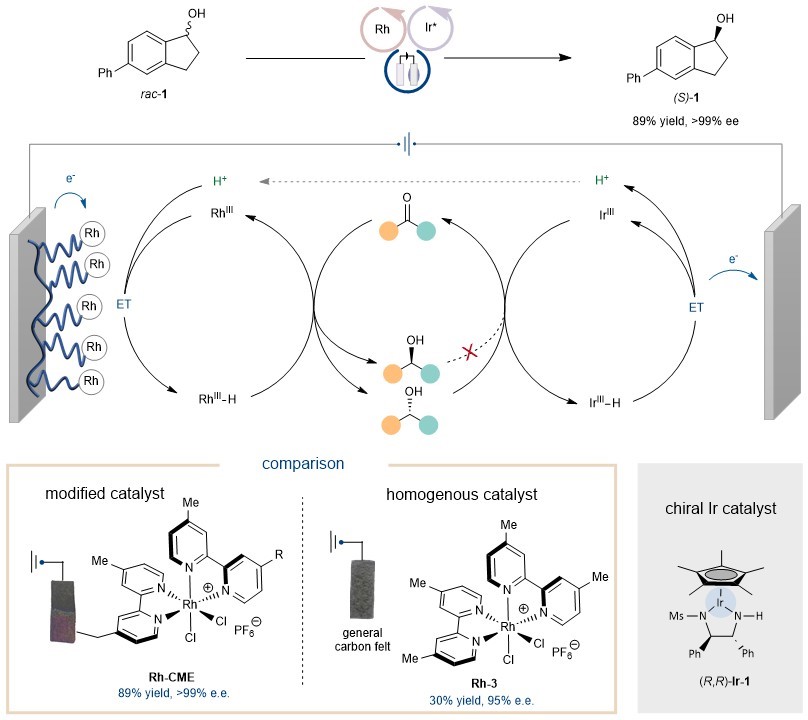
Figure 2. Comparison of chemically modified electrode and homogenous Rh catalysts
Although the same chiral iridium catalyst was used, the substrate applicability of the kinetic resolution strategy through air oxidation in the original literature (Angew. Chem. Int. Ed. 2008, 47, 2447) was limited (Figure 3). The researchers believe that even if the resolution selectivity of a single cycle is not satisfactory, multiple cycles can significantly increase the enantiomeric excess. Additionally, using oxygen as an oxidant results in more side reactions, while electro-oxidation is more selective.
In summary, the new strategy holds promise in transforming unsuccessful kinetic resolution reactions into successful deracemization reactions without changing the catalyst.
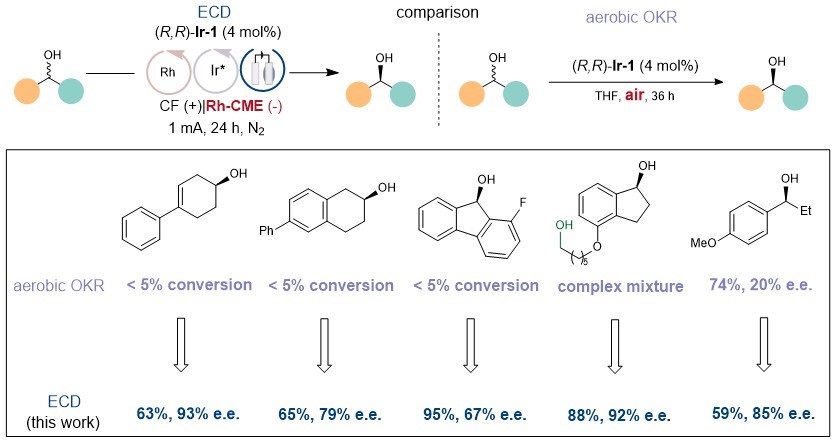
Figure 3. Comparison between the research team’s method and the previous method
Regarding mechanistic studies, the team initially demonstrated redox incompatibility through cyclic voltammetry experiments. Subsequently, through deuterium-labeling experiments, they proved that the source of Rh−H is the reduction of protons rather than the hydride transfer from Ir−H. This reaction also benefits from the general advantages of chemically modified electrodes. First, the electrodes can be reused; simply rinsing them with a solvent prepares them for the next experiment. Second, the catalyst loading is low. For a 2.0 mmol reaction, the catalyst loading was measured to be approximately 0.05 mol%. The recyclability and low loading characteristics increase the practicality of using chemically modified electrodes.
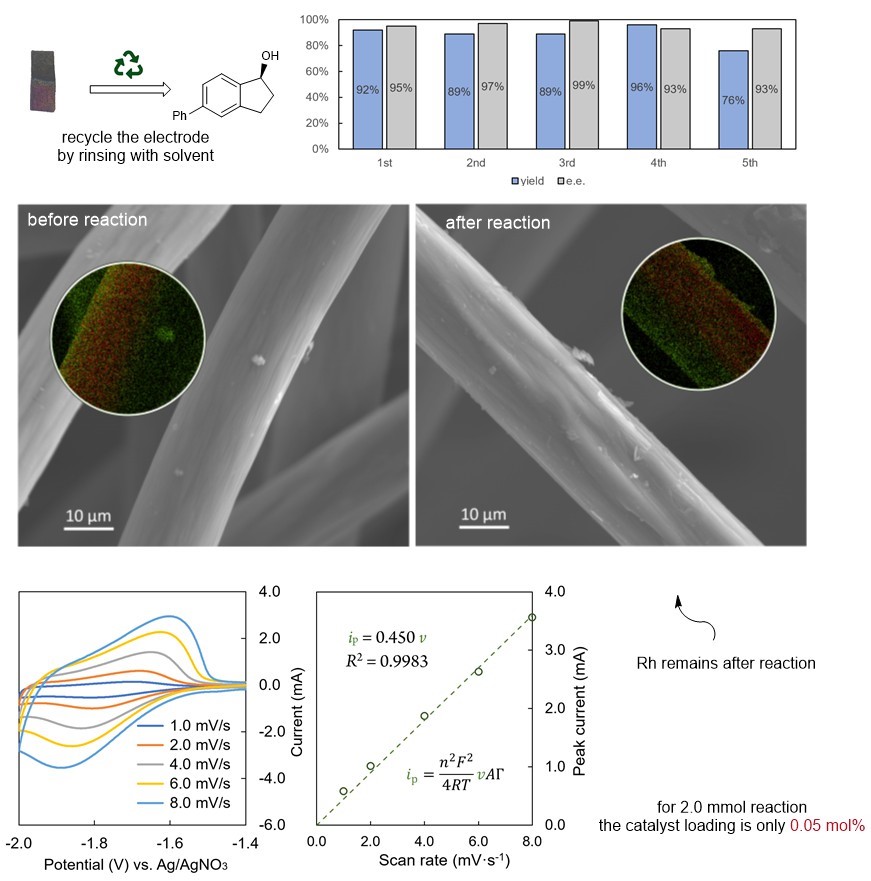
Figure 4. Recycle and characterization of chemically modified electrodes
In this work, Jianchun Wang’s team cleverly overcame the redox compatibility issue by employing a chemically modified electrode strategy, which is rarely explored in the field of organic chemistry. This allowed them to break the principle of microscopic reversibility and successfully achieve electrocatalytic cyclic deracemization. This method holds promise for providing a new approach to other contra-thermodynamic reactions.
Ph.D. student Cheng-Jie Zhu and master’s student Xiuying Yang are the first and second authors, respectively. Associate Professor Jianchun Wang is the corresponding author, and SUSTech is the sole corresponding institution.
This work was supported by the National Key R&D Program of the Ministry of Science and Technology, National Natural Science Foundation of China (NSFC), Guangdong Provincial Key Laboratory of Catalytic Chemistry, and the Shenzhen Science and Technology Innovation Commission.
Paper link: https://www.nature.com/articles/s41929-024-01189-2