Researchers make breakthrough in field of chiral sulfur chemistry
2024-09-16
Sulfur stereogenic molecules are widely present in natural products and have a significant impact on drug development (Fig. 1a). Among them, sulfilimines are chiral molecules bearing S(IV) stereocentres, which exhibit great value in chemistry and biology. However, much less is known about their selective synthesis, which in turn limits the exploration of their biological activity. Conventionally, enantioenriched sulfilimines are synthesised through enantioselective imination of sulfides, a process which predominantly relies on steric differentiation of the two S-substituents by a catalyst. While this strategy has successfully produced chiral aryl alkyl sulfilimines and dialkyl sulfilimines with high optical purity, the formation of enantiopure diaryl sulfilimines has been limited, presumably due to the small difference in the size of the two (hetero)aryl moieties (Fig. 1b). Therefore, developing a general enantioselective method to directly prepare chiral aryl sulfilimines with broad functional group compatibility and high levels of enantioselectivity remains an unsolved challenge.
Associate Professor Tiezheng Jia’s research team from the Department of Chemistry at the Southern University of Science and Technology (SUSTech) has pioneered novel and efficient organosulfur chemical synthesis methods using the Chan-Lam coupling reaction as the main tool. The Jia group developed an enantioselective copper-catalysed Chan-Lam coupling S-arylation of sulfenamides with arylboronic acids, providing a streamlined approach to synthesize diverse diaryl sulfilimines with a high level of chemoselectivity and stereoselectivity.
Their research work, entitled “Enantioselective Chan-Lam S-arylation of sulfenamides”, has been published in Nature Catalysis.
The vast majority of Chan-Lam coupling has a simple catalytic system without an external ligand, and lacks chiral factors that can be controlled. However, solvents, bases, or anions of catalysts can effectively promote the progress of the reaction (Fig. 1c), resulting in a racemic background reaction that is difficult to overcome, thereby limiting the application of Chan-Lam coupling in asymmetric catalysis. Based on previous work, the Jia group proposed a new strategy for constructing enantioenriched sulfilimines by enantioselective Chan-Lam S-arylation of sulfenamides. First, to overcome the racemic background reaction caused by substrate chelation and make it easier to introduce additional ligands in the catalytic process, they selected N-aryl sulfenamide without coordination as the substrate design. Secondly, developing ligands that can provide a well-defined chiral environment and achieve the dual control of excellent chemoselectivity and enantioselectivity was pivotal for achieving asymmetric S-arylation of sulfenamides and arylboronic acids.
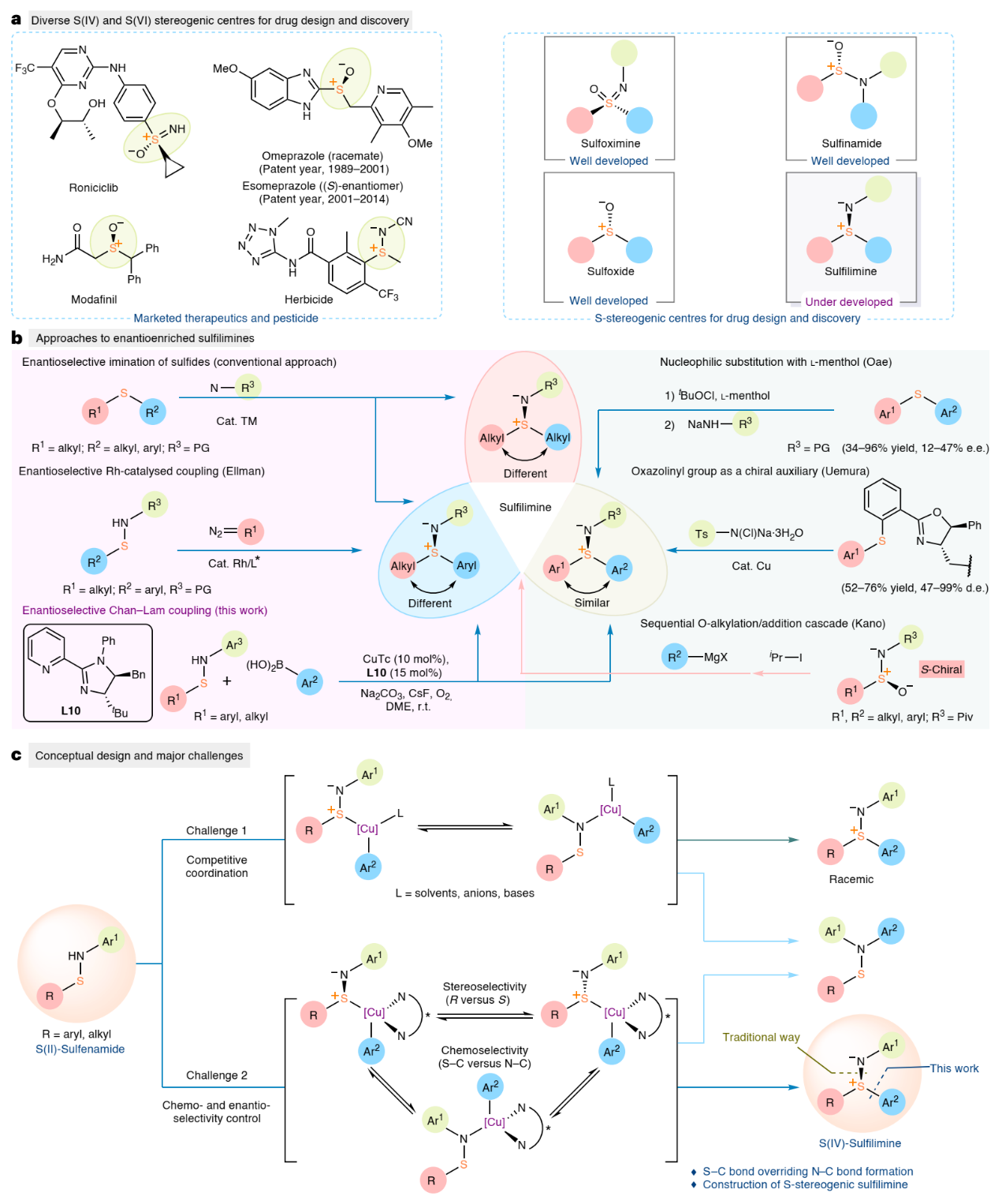
Figure 1. Background and conceptual design
The researchers developed a highly chemoselective and enantioselective Chan-Lam coupling of sulfenamides with arylboronic acids to synthesize a diverse range of diaryl and alkyl aryl sulfilimines containing a stereogenic sulfur center. A copper catalyst generated from the newly developed 2-pyridyl N-phenyl dihydroimidazole ligand enabled effective enantiocontrol through a well-defined chiral environment and high reactivity that outcompeted the racemic background transformation. With this strategy, a single chiral ligand can deliver either enantiomeric product by exchanging the aryl groups of the sulfenamide and arylboronic acid substrates, thereby circumventing the need to prepare the enantiomeric ligands from unnatural D-amino acids. The 2-pyridyl protecting group could be removed from the product, which can then be readily transformed to S(IV) and S(VI) derivatives with retained enantiopurity. Additionally, the synthetic utility of this asymmetric coupling was underscored by synthesising two sulfilimine-analogs of patented bioactive molecules.
Experimental data, supported by computational studies, revealed that the unconventional chemoselectivity favoring C-S bond over C-N bond was enabled by the disproportionation of Cu(II) complexes prior to the deprotonation of sulfenamides. Furthermore, the excellent enantioselectivity arose from entropy differences between transition states. The protocol represents a groundbreaking enantioselective Chan-Lam coupling approach, which is anticipated to serve as a powerful tool to prepare chiral scaffolds in medicinal chemistry and organic synthesis.
Postdoctoral fellow Qingjin Liang and Ph.D. student Xinping Zhang (experimental part), both from SUSTech, and postdoctoral fellow Madeline Rotella (theoretical calculation part) from UPenn, are the co-first authors of this paper. Associate Professor Tiezheng Jia and Professor Marisa Kozlowski from UPenn are the co-corresponding authors. Master Zeyu Xu from SUSTech also made important contributions to this work.
Paper link: https://doi.org/10.1038/s41929-024-01213-5




