Researchers develop modular enantioselective approach for boron-stereogenic BODIPYs
2024-09-29
BODIPY (boron dipyrromethene) is an N,N-π-conjugated tetracoordinate organoboron compound formed through the chelation of two pyrrole units. As an important fluorescent dye, BODIPYs are widely applied in the fields such as fluorescent probes, bioimaging, optoelectronic materials, and photocatalysts, owing to their excellent photophysical properties, good biocompatibility, and ease of synthesis and modification. However, BODIPYs featuring boron-stereogenic centres in enantioenriched forms are scarce, and their synthesis with structural diversity remains underdeveloped and challenging.
Current boron-stereogenic BODIPYs are significantly limited in structural diversity, and effective synthetic methods for their construction are highly restricted. Furthermore, the control of enantioselectivity on the boron centres has been a long-standing challenge due to the configurationally unstable nature of the tetracoordinate boron compounds. Given the increasing demand for chiroptical luminophores with versatility in chiral sensing and labeling, the development of efficient enantioselective methods for the construction of boron-stereogenic BODIPYs with structural diversity is highly attractive and desirable.

Associate Professor Chuan He’s research team from the Department of Chemistry at the Southern University of Science and Technology (SUSTech) has long been dedicated to the synthesis and application of chiral heteroatom (such as silicon, germanium, boron, sulfur, etc.) compounds. Building upon these previous studies, the He group demonstrated a modular enantioselective assembly of multi-substituted boron-stereogenic BODIPYs in high efficiency with excellent enantioselectivities.
Their research work, entitled “Modular enantioselective assembly of multi-substituted boron-stereogenic BODIPYs”, has been published in Nature Chemistry.
Structurally, the original achiral BODIPY core is assembled by two pyrrole units and complexed with a BF2 group, which provides a two-fold axis of symmetry and two mirror planes. Breaking this mirror symmetry is key to generate the boron-stereogenic centre, though achieving this synthetically has proved to be very challenging (Fig. 1a). Despite its simplicity and aesthetic appeal, BODIPY derivatives holds remarkable potential for structural diversity, with at least five sites that can be conveniently decorated with different functional substituents. The multi-substituted BODIPY featuring a boron-stereogenic centre would naturally increase its structural diversity in a three-dimensional space. However, due to the lack of general and efficient asymmetric synthetic methods, this potentially huge diversity of boron-stereogenic BODIPYs has not been exploited to date. To address the aforementioned challenges, the He group developed a modular assembly approach that can install substituents “at-will” onto the BODIPY core accurately (Fig. 1b).
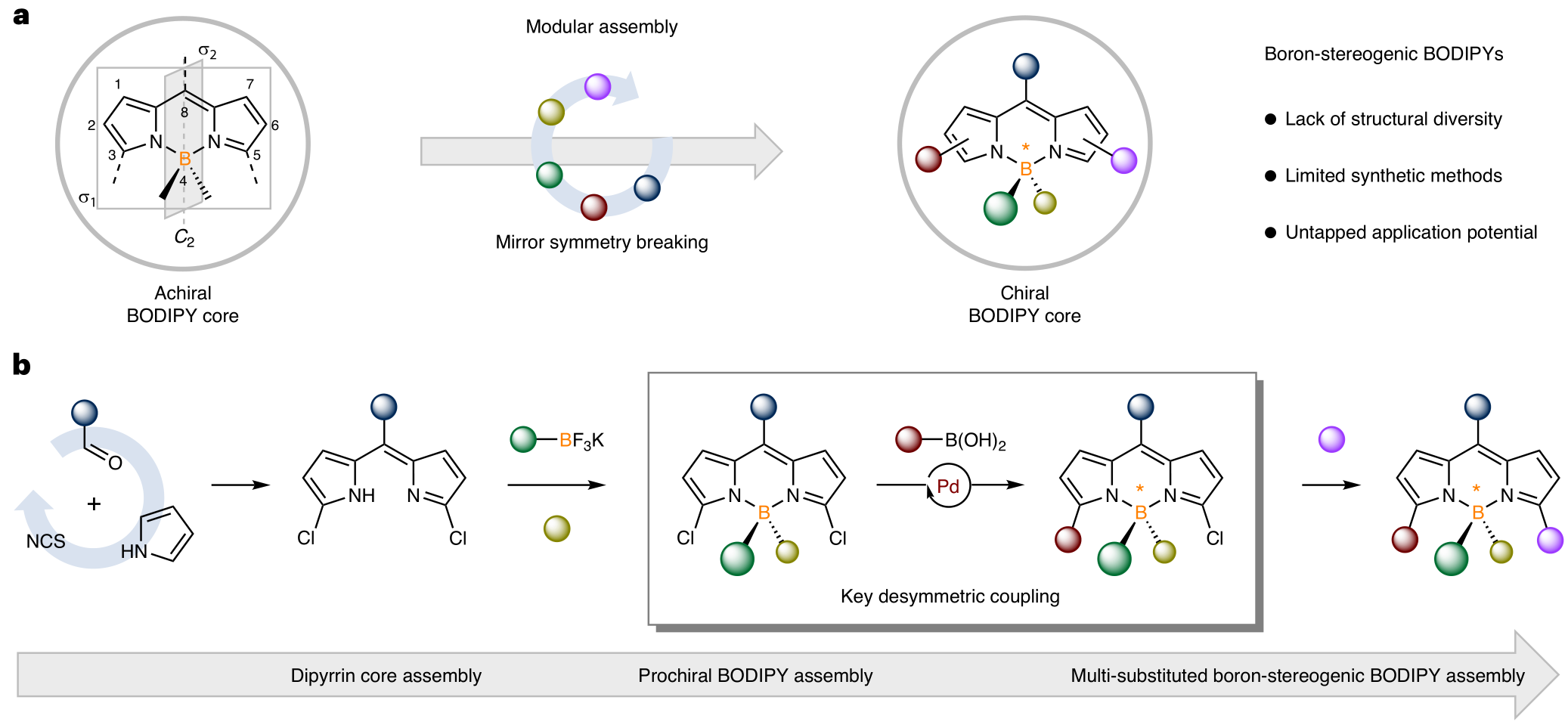 Figure 1. Modular enantioselective assembly of multiply boron-stereogenic BODIPYs
Figure 1. Modular enantioselective assembly of multiply boron-stereogenic BODIPYs
With the optimised conditions in place, the research team systematically investigated the substrate scope at five positions of the chiral BODIPY scaffold. At the C3 position, a variety of commercially available arylboronic acids, styrylboronic acids, alkynylboronic acid derivatives, and alkylboronic acids exhibited good compatibility under optimal conditions. At the C8 position, both electron-withdrawing and electron-donating aryl and heteroaryl groups were well tolerated. By modifying the B4 position with potassium trifluoroborate salts or post-functionalizing the B-F bonds, prechiral substrates with functionalization at this position could be rapidly obtained. These substrates led to the formation of a series of boron-stereogenic C-C-BODIPYs and C-O-BODIPYs.
Further post-functionalization of the C5 position in compound 3a allowed for the construction of structurally diverse boron-stereogenic BODIPY compounds with various functional group substitutions. The researchers also conducted detailed studies on the photophysical properties of these compounds, including absorption spectra, emission spectra, fluorescence quantum yields, and chiral luminescence.
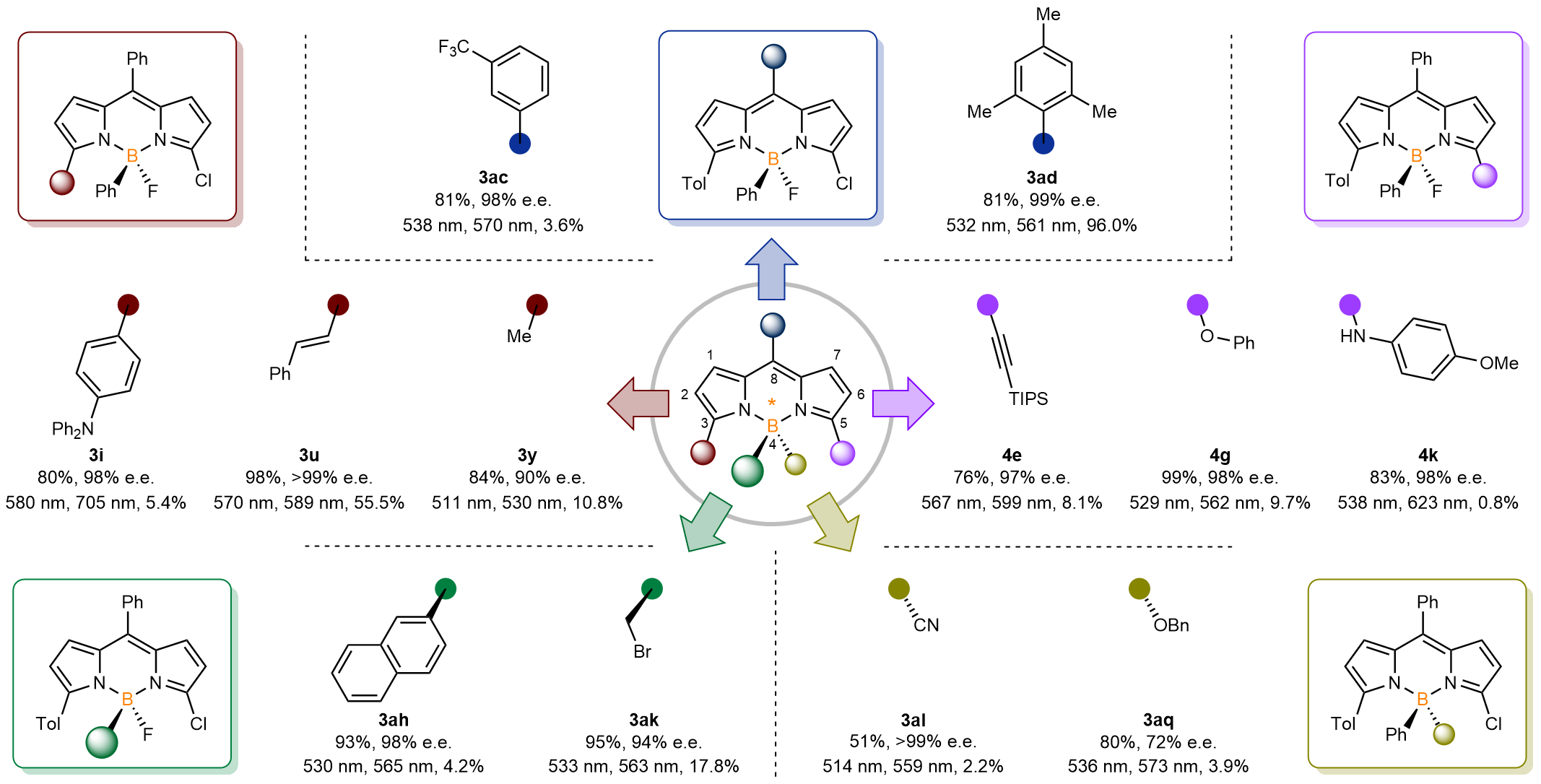 Figure 2. Substrate scope and post-functionalization enable modular assembly at five positions
Figure 2. Substrate scope and post-functionalization enable modular assembly at five positions
The researchers developed an efficient synthetic route for the modular enantioselective assembly of boron-stereogenic BODIPYs. Such an approach generated structural diversity in a programmable fashion, gaving access to a wide range of enantioenriched multi-substituted BODIPYs featuring various functionalities. This strategy is distinguished by its versatility, modularity, excellent enantioselectivity, and scalability. A key aspect to the success lies in the Pd/phosphoramidite-catalysed desymmetric Suzuki cross-coupling, allowing the precise discrimination of the two α C–Cl bonds of the designed prochiral BODIPY scaffold. The subsequent diverse elaborations further underpin the power and value of this synthetic methodology.
Photophysical properties and application in chiral recognition demonstrated the potential of these boron-stereogenic BODIPYs to be used as chiroptical luminophores. Additionally, the synthetic utility of this modular enantioselective protocol towards multi-substituted boron-stereogenic BODIPYs not only enriches the chemical space of chiroptical BODIPY dyes but also inspires the new aspects of chiral boron chemistry.
Ph.D. student Li-Qing Ren and Research Assistant Baoquan Zhan, both from SUSTech, are the co-first authors of this paper. Associate Professor Chuan He is the sole corresponding author. Master’s student Jiayi Zhao made important contributions to this work. Dr. Xiaoyong Chang provided guidance on single crystal diffraction, while master’s student Ruicong Feng assisted with photophysical measurements.
The above research was supported by the National Natural Science Foundation of China (NSFC), Guangdong Provincial Key Laboratory of Catalysis, Guangdong Pearl River Talent Program and the Shenzhen Science and Technology Innovation Committee. The researchers also acknowledge the SUSTech Core Research Facilities for providing technical support for the photophysical tests.
Paper link: https://www.nature.com/articles/s41557-024-01649-z