Critical role of Kindlin-2 in regulating adipogenesis and metabolism revealed by SUSTech undergrad
2019-08-20
Obesity is the fifth leading cause of human death worldwide. Every year at least 2.8 million adults worldwide die from obesity-related diseases. Obesity is often accompanied by hypertension, early signs of cardiovascular disease and other chronic diseases, such as insulin resistance, type 2 diabetes, fatty liver, atherosclerosis and degenerative diseases. Therefore, it is of great theoretical and clinical significance to study the molecular mechanism of obesity and related interventions.
Earlier this month, Southern University of Science and Technology (SUSTech) undergraduate student Guo Yuxi (Class of 2020) published a research paper entitled “Lipoatrophy and metabolic disturbance in mice with adipose-specific deletion of kindlin-2” in an internationally renowned journal JCI Insight. In this study, he and coworkers at SUSTech investigated the role of Kindlin-2, a key focal adhesion protein, in fat tissue formation and metabolism.
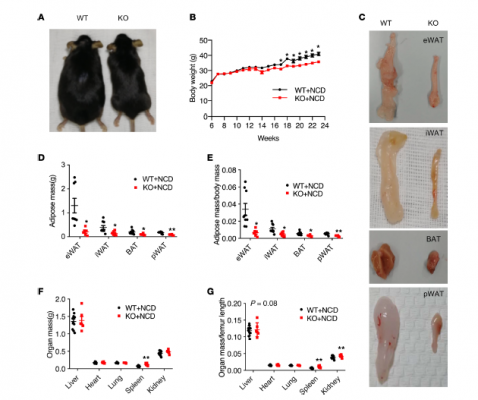
Adipose tissue plays a critical role in regulating energy balance and metabolism and its dysfunction is associated with metabolic diseases, such as obesity and insulin resistance. There are 2 main types of adipose in mammals, white adipose tissue (WAT) and brown adipose tissue (BAT). The former function primarily in energy storage, while the latter dissipates energy through heat to maintain normal body temperature.
The study initially examined the effects of Kindlin-2 loss on adipogenesis and glucose and fat metabolism under physiological and high-fat diet conditions using adipose tissue-specific knockout mice as experimental animal models. The results showed that Kindlin-2 specific deletion in adipose tissue resulted in a drastic loss of adipose tissue, including both white and brown fat, fatty liver formation, insulin resistance and type II diabetic manifestations.
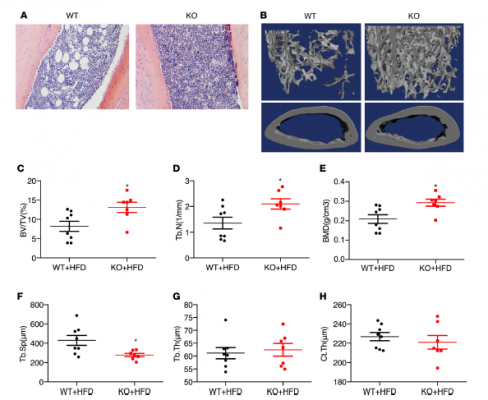
The authors demonstrated that Kindlin-2 deletion in adipose tissue caused a high bone mass phenotype in mice. The knowledge gained from this study will help scientists better understand the role of adipocyte-extracellular matrix (ECM) interaction and possible involvement of its abnormal alterations in pathogenesis of metabolic diseases. It may provide a new target for the prevention and treatment of metabolic diseases, such as diabetes.
Gao Huanqing (postdoctoral fellow), Guo Yuxi, and Yan Qinnan (research assistant) are co-first authors and professors Xiao Guozhi and Cao Huiling are co-corresponding authors of this paper.
This study was supported, in part, by the National Natural Science Foundation of China (NSFC), the Guangdong Provincial Science and Technology Innovation Council, and Science and Technology Innovation Commission of Shenzhen Municipal Government.
Original article: https://insight.jci.org/articles/view/128405




