Synthesis of natural alkaloids becomes more efficient under SUSTech research
2019-06-03
The Daphniphyllum alkaloids are a structurally fascinating and remarkably diverse family of natural products. General strategies for the chemical synthesis of their challenging architectures are highly desirable for efficiently accessing these intriguing alkaloids and addressing their pharmaceutical potential.
Recently, Associate Professor Xu Jing from the Department of Chemistry and the Shenzhen Grubbs Institute at Southern University of Science and Technology (SUSTech) successfully led his research team to complete the efficient asymmetric synthesis of Himalensine A through a 14-step route. This important development was published in Angew. Chem. Int. Ed., under the title of “A Concise Synthesis of (-)-Himalensine A.”

Among the complex small molecule natural products that are so vast, the alkaloids are a special type of existence. These alkaloids are isolated from plants of the genus Daphniphyllum that are widely distributed in China, New Guinea, and Japan, and have a highly complex chemical structure. Traditional Chinese medicine believes that the plants of this genus can clear away heat, detoxify, promote blood circulation, relieve phlegm, relieve pain, and treat sores and swollen limbs, among other alleged maladies. At the same time, modern research has found that some of the alkaloids have good anti-cancer, anti-HIV and neurotrophic activities. The complex and diverse chemical skeleton structure and promising biological activity of the Daphniphyllum alkaloids have long attracted the attention of numerous synthetic chemists.
Himalensine A, a unique member of the calyciphylline A‐type subfamily, was isolated from Nepalese Daphniphyllum himalense by Chinese Academician Yue Jianmin of the Shanghai Institute of Materia Medica, Chinese Academy of Sciences (SIMM-CAS) in 2016. While its bioactivity remains unexplored, himalensine A contains a highly congested pentacyclic ring system that includes a unique 2‐azabicyclo[3.3.1]nonane structure with six stereogenic centers, and a quaternary carbon center. This creates significant synthetic challenges for organic chemists. In 2017, the Dixon team at the University of Oxford successfully completed the asymmetric total synthesis of Himalensine A for the first time in a 22-step route.
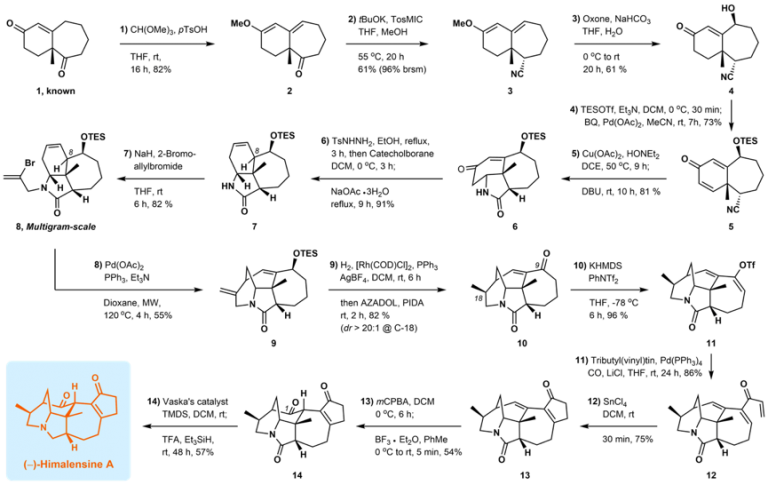
The new route found by the research group of Professor Xu Jing utilizes an efficient molecular skeleton construction strategy, rapid and selective introduction of functional groups, and minimizes the use of protecting groups. Their 14-step synthesis provides new ideas for the efficient synthesis of more complex Daphniphyllum alkaloids.
SUSTech is the first communication affiliation, and Associate Professor Xu Jing is the sole corresponding author. The first author is SUSTech Ph.D. candidate Chen Yuye. Fellow doctoral candidates Hu Jingping and Dr. Guo Liandong worked with research assistant Zhong Weihe and research assistant professor Ning Chengqing in making important contributions to the article.
Professor Xu Jing’s research team thanked the National Natural Science Foundation of China, the Shenzhen Municipal Science and Technology Commission, the Shenzhen Development and Reform Commission, SUSTech and the Shenzhen Grubbs Institute for their support.
Article link: https://onlinelibrary.wiley.com/doi/10.1002/anie.201902908




