Researchers gain new insights into the origin of chiral molecular selectivity
2020-05-08
Recent research led by Southern University of Science and Technology has possibly solved a long-held question that has thwarted chemists for generations: Where did the first molecule with chiral selectivity come from?
Associate Professor Yanggang Wang (Chemistry) led his research team, in conjunction with collaborators at Sichuan University and Hong Kong Baptist University, to publish a ground-breaking paper in the high-impact academic journal Nature Chemistry (IF = 23.19). The paper was titled, “Enantioselective photoinduced cyclodimerization of a prochiral anthracene derivative adsorbed on helical metal nanostructures.”
The molecular chirality is widespread through nature. Without the influence of other chiral centers, a pair of chiral molecules (enantiomers) in mirror relationship with each other have the same free energy and equal generation probability.
However, biofunctional molecules, such as amino acids and DNA, usually show highly selective chirality in nature. Understanding how to synthesize a specific chiral structure in a pair of enantiomers would dramatically improve the synthetic development of drugs and advanced materials.
Usually, experiments show that chiral molecular selectivity (enantioselectivity) comes from the existing molecular chiral inductor used in the synthesis system. Can we obtain chiral molecular selectivity from the macro-rotation and structures in the absence of any molecular chiral inductors?
The research team produced chiral nanohelixes by glancing angle deposition onto a substrate that would rotate in either a clockwise or counterclockwise direction. A prochiral molecule, 2-anthracenecarboxylic acid (AC), is stereoselectively absorbed on the metal nanohelix, as an organic compound where two identical molecules are bound together known as a dimer.
Their experiments showed that the AC prochiral molecules absorbed on the metal nanohelix produce a photodimerization effect with a specific stereoselectivity. It shows that it is possible to control the chirality of the metal nanohelix by controlling the rotational direction of the substrate and ultimately selectively synthesize microscopic chiral molecules.
Even with the experimental observations, there are still questions about how the rotation of the substrate leads to chiral molecular selectivity (enantioselectivity) and how it is transmitted from the macro-level to the micro-level (or molecular level). The researchers found that the wavelike-chiral lattice on the metal nanohelix plays a vital role. These wavelike-chiral lattices consist of chiral periodic atomic fluctuations, instead of non-chiral flat planes, that can be optimally designed for AC absorption and dimerization (Figure 1).
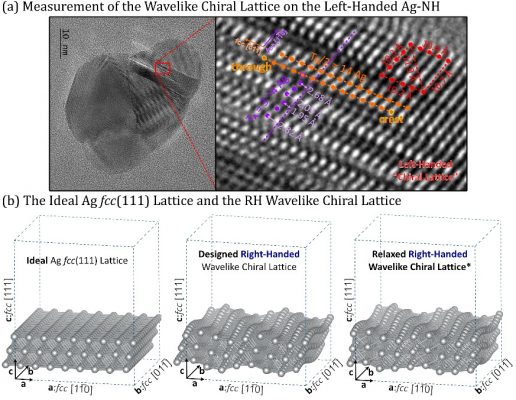
Figure 1. HRTEM image analysis and measurement of left-handed silver nanohelix; design, construction, and optimization of the theoretical model of the surface of right-handed wavelike-chiral lattice surface
On the right-handed wavelike-chiral lattice, the AC monomer has two different absorption configurations, ACf,- and ACf, (Figure 2). These two configurations can be transformed by crossing an overturned energy barrier.
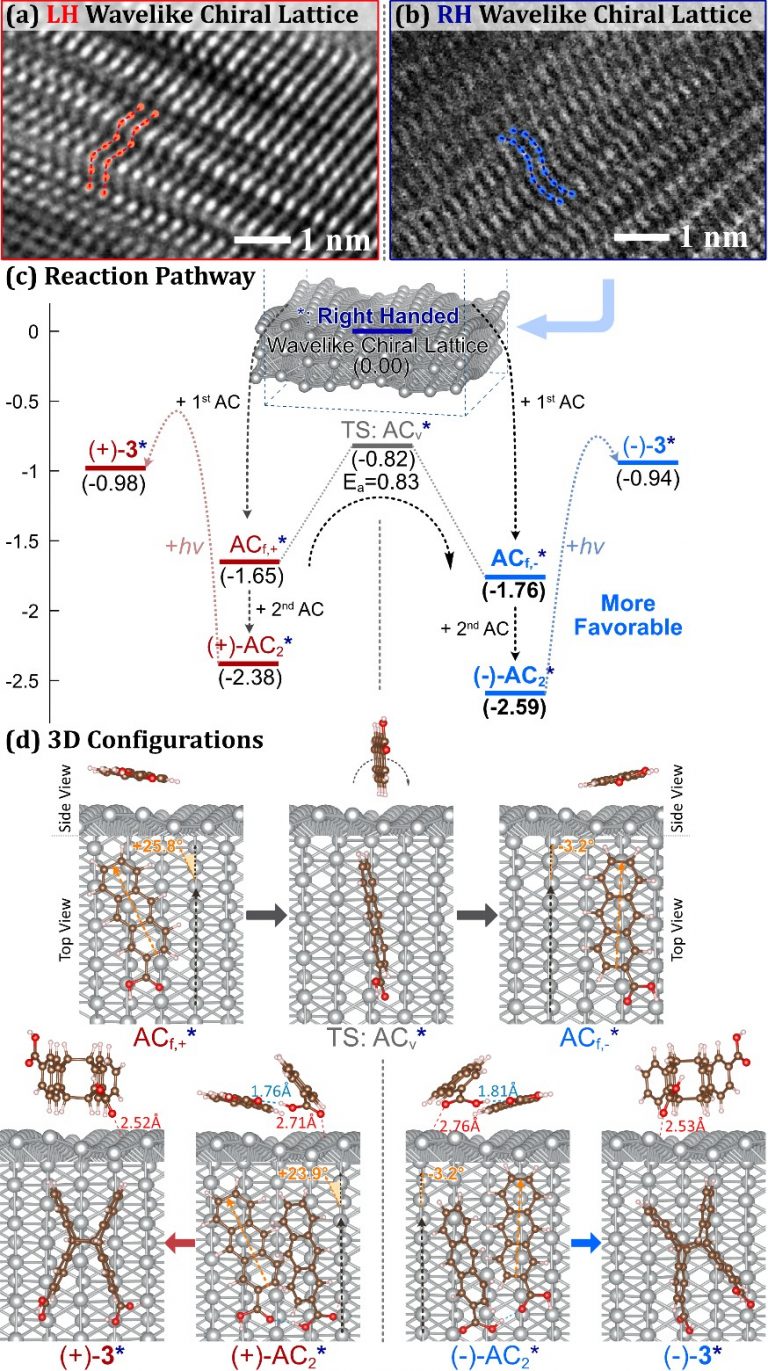 Figure 2. Comparison of HRTEM images of left-handed and right-handed silver nanohelix; theoretical calculation of AC molecular adsorption configuration, energy and reaction path Calculations show that right-handed silver nanoparticles have strong chiral selectivity for the adsorption of AC monomers and dimers, resulting in the chiral selection of the final product dimer product (-)-3
Figure 2. Comparison of HRTEM images of left-handed and right-handed silver nanohelix; theoretical calculation of AC molecular adsorption configuration, energy and reaction path Calculations show that right-handed silver nanoparticles have strong chiral selectivity for the adsorption of AC monomers and dimers, resulting in the chiral selection of the final product dimer product (-)-3
On the surface of the right-handed wavelike-chiral lattice, the lying AC orientation of (-)-AC2 is better aligned and results in a stronger surface electron exchange (see Figure 3). It results in a significantly higher concentration of (-)-AC2 absorbed on the surface than ( )-AC2 before photoinduced cyclodimerization. The difference in adsorption energy of AC dimer on the wavelike-chiral lattice transmits its chirality into microscopic organic molecules through the inorganic chiral lattice.
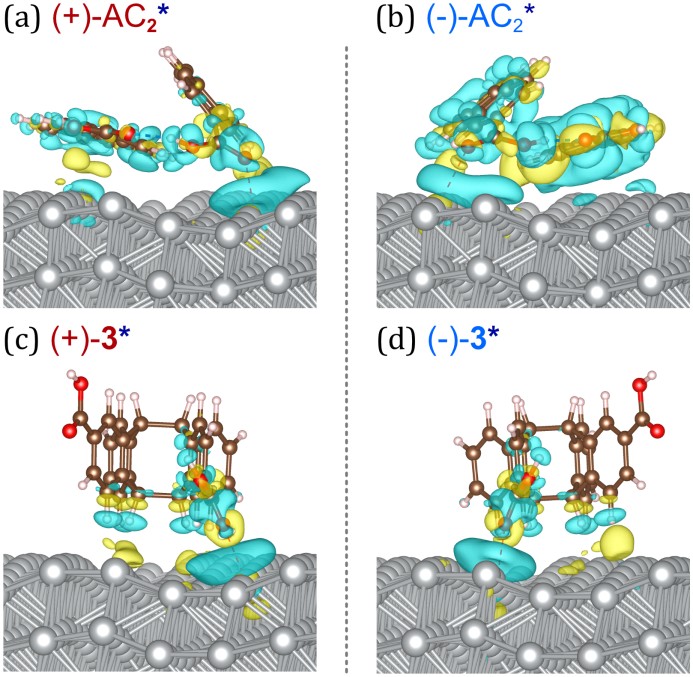 Figure 3. The electronic interaction of the chiral AC dimer with the surface of the right-handed wavelike-chiral lattice during adsorption
Figure 3. The electronic interaction of the chiral AC dimer with the surface of the right-handed wavelike-chiral lattice during adsorption
The paper proves that chemists can manipulate molecular chirality through macro-rotation, showing a new top-down strategy to regulate molecular chirality while providing new models for the origin of chirality, better known as chiral enantioselectivity.
SUSTech postdoctoral researcher Xia Guangjie was a co-first author of the paper, with Dr. Xueqin Wei (Sichuan University) and Dr. Junjun Liu (Hong Kong Baptist University). Associate Professor Yanggang Wang, Professor Cheng Yang (Sichuan University), and Associate Professor Zhifeng Huang (Hong Kong Baptist University) were the co-corresponding authors. Additional contributions came from the Department of Materials Science and Engineering (SUSTech).
The research received financial support from the National Natural Science Foundation of China, and the National Key Research and Development Program of China. The computational works are implemented on Taiyi HPC from the Center for Computational Science and Engineering at SUSTech.
Original link: https://www.nature.com/articles/s41557-020-0453-0




