Single-atom catalysis could change storage of renewable energy
2020-05-18
Electrolyzed water is known as a highly effective method of storing renewable energy, and new methods for single-atom catalysis could see significant changes to the industry as a whole.
Associate Professor Meng Gu (Materials Science and Engineering) and Associate Professor Hu Xu (Physics) worked with their colleagues at Oregon State University (Chemical, Biological, and Environmental Engineering) to publish a breakthrough paper on single-atom catalysis.
Their paper was published in the high-impact academic journal, Journal of the American Chemical Society (JACS), under the title of “Ultrahigh-Loading of Ir Single Atoms on NiO Matrix to Dramatically Enhance Oxygen Evolution Reaction.”
The existing challenge behind using electrolyzed water is that the 4-electron process of the oxygen evolution reaction (OER) is too slow. It significantly reduces electrolysis efficiency and energy utilization. Most commercial catalysts used in OERs are based on precious such as iridium oxide (IrO2) and ruthenium oxide (RuO2). However, they are very expensive, hindering their large-scale application. It is with this in mind that single-atom catalysis has been the focus of considerable research, due to their many beneficial features.
The researchers provided a simple and highly effective method for producing ultra-high Ir-loaded nickel oxide (NiO) catalysts, bringing the volume of single Ir atoms in NiO to 18 wt%. It represents the proportion of iridium in the nickel oxide catalyst – a previously unprecedented weight compared to the entire weight of the catalyst).
The current density of the OER under an overpotential of 260 mV is 46 times that of IrO2 and 57 times that of unloaded single atom NiO. The structure and performance of the catalyst did not change after ten hours of OER, indicating high stability. The researchers used density functional theory (DFT) calculations to determine that the substituted Ir atom can significantly improve the OER performance of NiO.
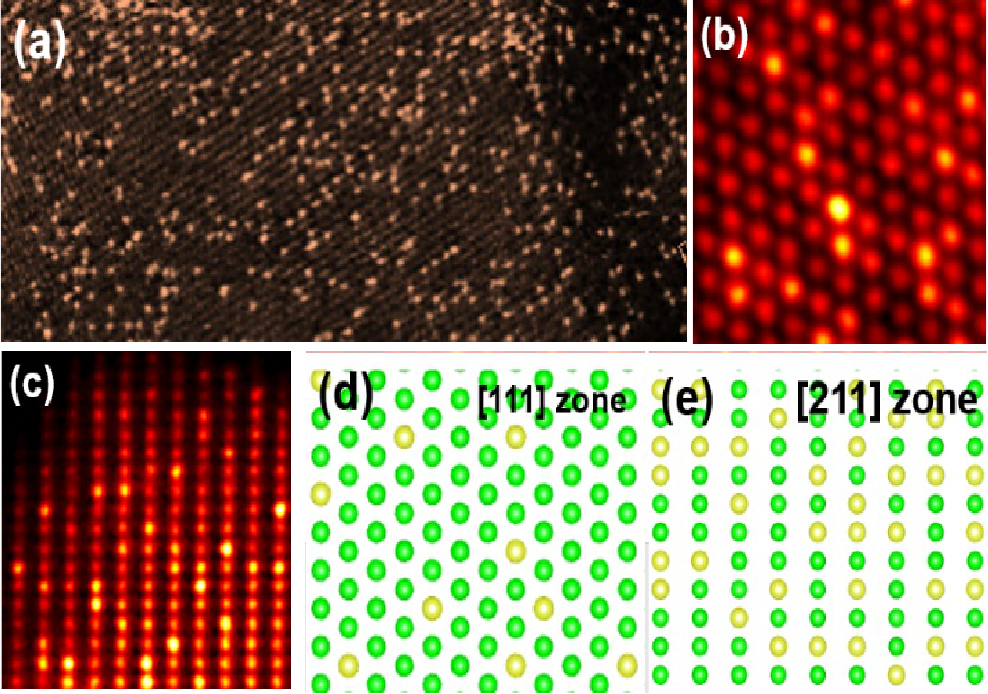 Fig.1 Transmission electron micrograph of spherical aberration correction of high-density iridium single atoms on the surface of nickel oxide
Fig.1 Transmission electron micrograph of spherical aberration correction of high-density iridium single atoms on the surface of nickel oxide
The researchers have not only produced a simple, stable, and highly active OER catalyst but provided a catalyst with the highest load among similar materials thus far. Their study also showed that other metals such as manganese (Mn), iron (Fe), cobalt (Co) and platinum (Pt) could all be used in NiO catalysts, representing high levels of flexibility of single-atom catalysts.
Associate Professor Meng Gu said that due to the practical and large-scale advantages of the catalysts, it has vastly simplified the process, providing significant flexibility that will suit commercial production. It has also accelerated the pace of high-performance, low-cost electrolyzed water catalysts, which should reduce the energy consumption and energy utilization of the process.
The co-first authors of the paper were postdoctoral researchers Qi Wang, Xiang Huang, and Zhiliang Zhao. The co-corresponding authors were Associate Professor Meng Gu, Associate Professor Hu Xu, and Oregon State University Assistant Professor Zhenxing Feng.
This work was supported by the National Natural Science Foundation of China, Guangdong Innovative and Entrepreneurial Research Team Program, Shenzhen Peacock Plan, Shenzhen DRC project, Guangdong Provincial Key Laboratory of Energy Materials for Electric Power, and Shenzhen Clean Energy Research Institute. XAS measurements were done at 9-BM at Advanced Photon Source of Argonne National Laboratory. The use of APS of ANL is supported by the DOE. This work was supported by the Pico Center at SUSTech CRF, which receives support from the Presidential Fund and Development and Reform Commission of Shenzhen Municipality.
At the same time the JACS paper was published, the high-impact academic journal Nature Catalysis published a review of Associate Professor Meng Gu’s earlier paper. His group’s paper was titled, “Reversible loss of core-shell structure for Ni–Au bimetallic nanoparticles during CO2 hydrogenation,” with the review article called, “The dynamics of the peel.”
The author of the review believes that the catalytic activity of a material is closely related to its surface atoms and electronic structure. In most cases, it is assumed that the catalyst retains most of its initial state characteristics during the reaction, especially if the catalyst structure is the same before and after the reaction. He argues that significant changes during the reaction would be ignored if attention is only paid to the catalyst before and after the reaction.
Due to the dramatic environmental changes during the reaction, the surface structure of the catalyst can change dramatically. It is challenging to characterize the changes in the surface structure under real reaction conditions. However, Associate Professor Meng Gu’s paper has provided insights into the reaction mechanism and how to apply in-situ characterization methods.




