New progress in silicon-centered and sulfur-centered chiral compounds construction
2020-08-08
The development of new chemical transformations based on asymmetric catalysis has been the focus of significant research across the world. Starting from simple raw materials, recent progress by scholars at Southern University of Science and Technology (SUSTech) has made new developments in the concise construction of silicon-centered and sulfur-centered chiral compounds.
Their progress will lay the foundation for the continued development and applications in fields such as medical science, chiral functional materials, and electronic devices.
Associate Professor Chuan He (Chemistry) has led his research group to make significant progress in the construction of silicon-stereogenic silanes and sulfur-stereogenic sulfoxides. Their research outcomes were published in the high-impact academic journals, Journal of the American Chemical Society (JACS) (IF = 14.612) and ACS Catalysis (ACS Catal.) (IF = 12.35).
Silicon constitutes approximately 28% of the mass of Earth’s crust, but organic silicon has been omitted from biological evolution. Therefore, chiral organosilicon compounds are not found in nature. With silicon sitting directly under carbon on the periodic table, it claims to be an alternative to organic carbon. It has left people wondering whether there is a parallel silicon-based life world out of our carbon-based life world.
In their paper published in JACS called “Streamlined Construction of Silicon-Stereogenic Silanes by Tandem Enantioselective C–H Silylation/Alkene Hydrosilylation,” the creation of silicon-stereogenic centers in enantioenriched forms have been explored far less due to their intrinsic properties. The increasing demand for novel silicon-stereogenic silanes has continued to drive the development of practical catalytic methods with high enantioselectivity for the synthesis of these compounds.
The research team developed a unique enantioselective C–H silyation/alkene hydrosilylation methodology. It combines readily available dihydrosilanes and alkenes to construct complex and diverse silanes in a single step with good to excellent yields and enantioselectivities. The silicon-stereogenic silanes were generated with operational simplicity, efficacy, and broad scope, which will find significant use in synthetic chemistry, medicinal chemistry, and materials science.
The co-first authors are Dr. Delong Mu and Dr. Wei Yuan. Associate Professor Chuan He is the sole correspondent author with SUSTech as the only corresponding unit. Additional contributions came from the Shenzhen Grubbs Insitute. They received financial support from the National Natural Science Foundation of China, the Thousand Talents Program for Young Scholars, the start-up fund from Southern University of Science and Technology, the Shenzhen Nobel Prize Scientists Laboratory Project, Guangdong Provincial Key Laboratory of Catalysis, the China Postdoctoral Science Foundation, and Shenzhen Clean Energy Research Institute.
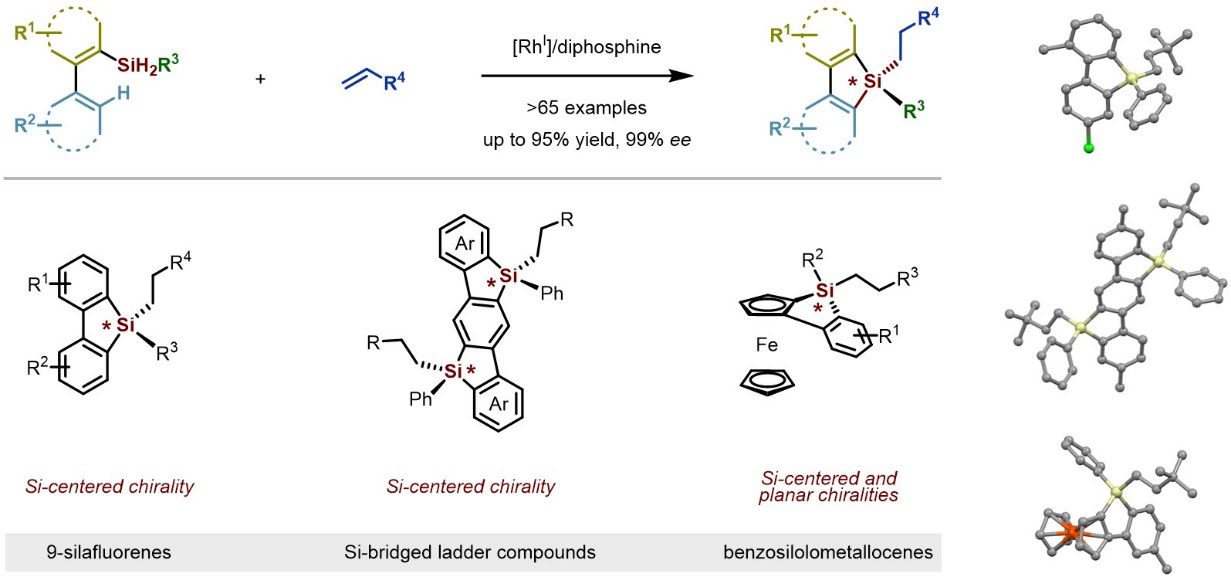 Figure 1. Streamlined Construction of Silicon-Stereogenic Silanes
Figure 1. Streamlined Construction of Silicon-Stereogenic Silanes
The paper published in ACS Catal was titled, “Dual-Ligand-Enabled Ir(III)-Catalyzed Enantioselective C–H Amidation for the Synthesis of Chiral Sulfoxides.” The research team developed a novel dual ligand system for chiral Ir(III) catalysis, providing a general, efficient, and straightforward way to construct sulfur chiral centers.
Chiral sulfoxides, in their enantiopure form, are central to many commercial pharmaceutical and bioactive compounds. They have also been used as efficient and versatile auxiliaries or ligand in asymmetric catalysis because of the stability. Traditional synthesis strategies for enantioenriched sulfoxides are still limited in their scope and efficiency.
The research team developed a novel Ir(III)-catalyzed enantioselective C–H amidation of dibenzyl sulfoxide (Figure 2). Their use of iridium (Ir) complex combined with a t-butyl cyclopentadienyl ligand and a modified chiral proline enabled the highly enantioselective sulfoxide-steered C–H bond activation. It provided an efficient and straightforward way to construct sulfur chiral centers with high yields and enantioselectivities.
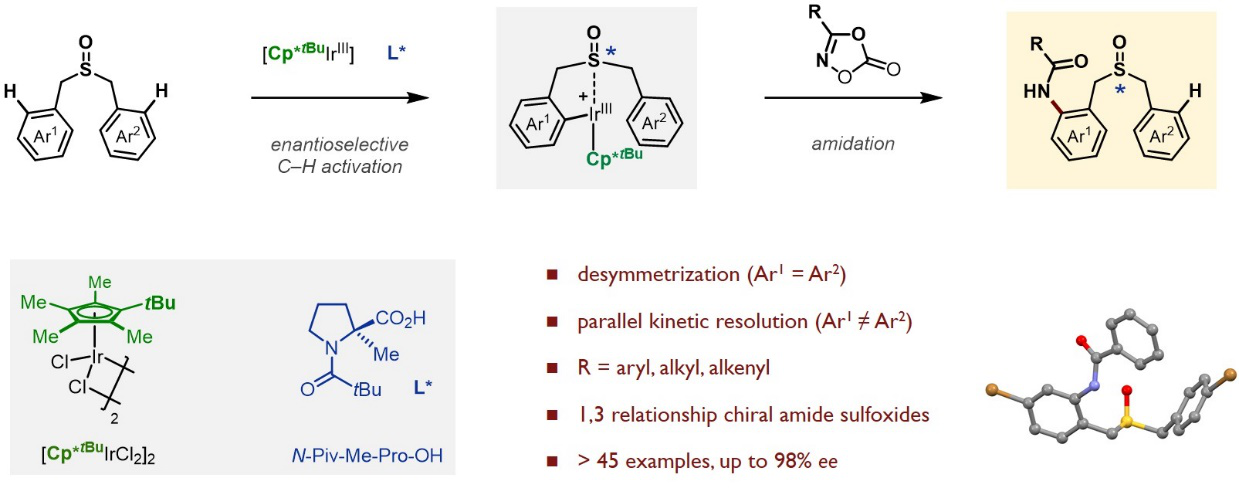 Figure 2. Enantioselective C–H Amidation for the Synthesis of Chiral Sulfoxides
Figure 2. Enantioselective C–H Amidation for the Synthesis of Chiral Sulfoxides
The first author of this paper is Dr. Wentan Liu. Associate Professor Chuan He is the sole corresponding author, with SUSTech as the sole communication unit. We are grateful for financial support from the National Natural Science Foundation of China, the Thousand Talents Program for Young Scholars, the start-up fund from Southern University of Science and Technology, the Shenzhen Nobel Prize Scientists Laboratory Project, and Guangdong Provincial Key Laboratory of Catalysis.




