SUSTech researchers achieve the first total synthesis of a rare and highly strained natural product
Department of Chemistry 2020-12-07
The biological study of highly strained natural products with significant phytotoxic, antibiotic, and apoptotic activity and anticancer function has been a major challenge for decades due to its natural scarcity. Recently, SUSTech Prof. LI Chuang-Chuang’s group at Department of Chemistry achieved the first total synthesis of a rare and highly strained natural product, with their research paper “Asymmetric Total Synthesis of the Highly Strained 4β-Acetoxyprobotryane-9β, 15α-diol” published in a top journal (Journal of the American Chemical Society, JACS).

The paper reported the total synthesis of a rare and highly strained natural product, 4b-acetoxyprobotryane-9β,15a-diol. This work created two firsts in synthetic chemistry: 1. RhI catalyzed [4 2] cycloaddition was first applied in natural product synthesis. 2. The highly strained trans-fused bicyclo[3.3.0]octane was constructed by a unique benzilic acid-type rearrangement for the first time.
This work was dedicated to the 10th anniversary of Southern University of Science and Technology.
4β-Acetoxyprobotryane-9β,15α-diol was the metabolites of Botrytis Cinerea. Botrytis Cinerea was the pathogen of more than 235 plant species and it was found all over the world. More than 70 natural products isolated from its metabolites have shown significant phytotoxic, antibiotic, and apoptotic activity. However, a further biological study was limited by its natural scarcity.
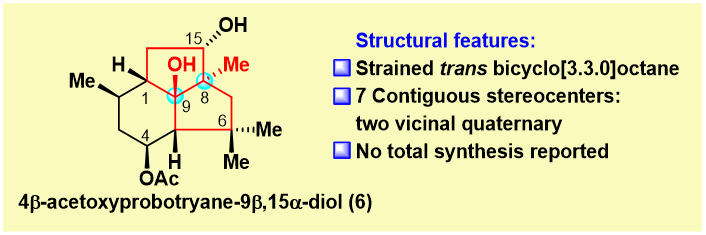
4β-Acetoxyprobotryane-9β, 15-diol was isolated by Collado and co-workers in 2001 from a culture of Botrytis cinerea. Structurally, it contains a sterically compact [6-5-5] tricyclic skeleton, as well as a highly strained trans-fused bicyclo[3.3.0]octane ring system. Of particular interest, “6” in the figure possesses seven contiguous stereocenters, including two vicinal quaternary centers (C8 and C9). This makes it a particularly challenging synthetic target. Strained natural products are important in medicinal chemistry, such as Taxol, a well-known anticancer drug. Strained natural products have good pharmacological capabilities and can bind to the desired biological targets tightly and selectively. Highly strained trans-fused bicyclo[3.3.0]octane was the potential gold mine for drug discovery. But the synthesis of its all-carbon format was only reported with 3 examples. Thus, the total synthesis of our target molecule holds both tremendous challenges and potential applications.

Starting with compound 11, the [4 2]cycloaddition precursor was synthesized in 2 steps. Subsequent RhI catalyzed [4 2]cycloaddition was achieved with excellent selectivity after optimizations. Another few steps were taken to approach the tricyclic compound 25. A unique benzilic acid-type rearrangement was discovered then, and the presence of TBAFO2 would promote benzilic acid-type rearrangement to form the highly strained trans-fused bicyclo[3.3.0]octane. To the best of our knowledge, this work represents the first example of a benzilic acid-type rearrangement promoted by TBAF. It is also the mildest and easiest approach. Finally, by removing the additional ester group, the first total synthesis of 4β-Acetoxyprobotryane-9β,15-diol (6) was completed.
The first and asymmetric total synthesis of the highly strained 4-acetoxyprobotryane-9,15-diol (6) was achieved via a linear sequence of 14 steps from the readily available compound 11, which explored a brand new approach to the highly strained trans-fused bicyclo[3.3.0]octane laid a foundation for future biological and medicinal studies.
SUSTechHKUST Joint Ph.D. graduate (2020) ZHANG Wen is the first author of this paper. Prof. LI Chuang-Chuang is the corresponding author of this paper. Prof. DAI Wei-Min from HKUST is the co-author. Besides, SUSTech Master graduate (2020) ZHOU Zi-Xiong, visiting student ZHU Xu-Jiang and visiting scholar SUN Zhang-Hua also made contributions to this paper.
The research papers received funding from the Leading Talents of the Special Support Program of the Central Organization Department, the National Natural Science Foundation of China, the Shenzhen Science and Technology Innovation Commission, and SUSTech.
Prof. LI Chuang-Chuang’team was devoted to the total synthesis of complicated natural products with unique structures and important bioactivity. Unique methods and strategies were developed by his lab, such as Type II [5 2] cycloaddition (Acc. Chem. Res., 2020, 53, 703). In this way, target molecules and their derivatives could be prepared rapidly from them and synthesized molecules’ bioactivity can be evaluated to develop potential drugs.
Paper link: https://pubs.acs.org/doi/pdf/10.1021/jacs.0c10116
Li Group: https://li.chem.sustech.edu.cn/




