Chinese scientists make the first probe of electronic angular momentum in chemical reaction
Pei WANG, Ransheng WANG 2021-02-26
Molecular reaction dynamics is the study of chemical and physical transformations of matter at the molecular level. Recently, scientists from China made substantial scientific progress in this area, by revealing the electronic angular momentum effect to a chemical reaction for the first time. Their work shows that a chemical reaction can be understood in detail at the quantum state-resolved level, through a combined study of molecular crossed beam experiments and theoretical quantum molecular reaction dynamics simulations.
Science, a high-impact journal, published this remarkable finding online on Feb. 26, 2021, entitled “Quantum interference between spin-orbit split partial waves in the F HD→HF D reaction.” Combining the results of the cross molecular beam experimental device with the quantum molecular dynamics theory simulation is the basic method to study the Molecular reaction dynamics.
In recent years, Prof. Xueming Yang from the College of Science at the Southern University of Science and Technology (SUSTech), in collaboration with the team of Prof. Xing’an Wang at the University of Science and Technology of China (USTC), has developed a more advanced molecular crossed beam apparatus with threshold ionization velocity map imaging technique. This method allows the probe to scattering product with a high angular resolution with quantum rotational-state recognition.
With such a powerful apparatus, Prof. Yang’s team has made a breakthrough in discovering quantum interference in chemical reactions, which mirrors the geometric phase effect. And the research progress was published on the Science in 2018, titled “Quantum interference in H HD→H2 D between direct abstraction and roaming insertion pathways.”
By building on previous studies and combining the new quantum reactive scattering theory developed by Prof. Zhigang Sun from Dalian Institute of Chemical Physics of the Chinese Academy of Sciences (DICP ), which included the electronic angular momentum effect, the joint teams successfully reported a combined experimental and theoretical study on the effect of electron spin and orbital angular momentum in the F HD → HF D reaction.
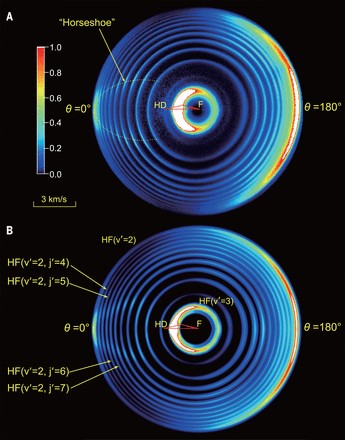 Figure 1. The D-atom product from the F HD → HF D reaction at a collision energy of 2.10 kcal/mol
Figure 1. The D-atom product from the F HD → HF D reaction at a collision energy of 2.10 kcal/mol
There is distinguished reactive scattering quantum resonance in the F HD (the Fluorine atom with the HD isotope of the H2 molecule) reaction. It has been taken as the prototype to resolve partial wave resonance structures in a chemical reaction.
With this feature, the scientists thought that the role of the electronic angular momentum of the F atom in this chemical reaction would be recognized. The F atom was characterized by a p electron orbit with l=1, which could influence the partial wave resonance structures.
It was found that, by including the electronic angular momentum, the single partial wave structure would split into a four-fold partial wave resonance structure, which was capable of varying the angular distributions of the chemical product. The energy of the electronic angular momentum is much smaller than the rotational energy of a diatomic molecule (~ several tens wavenumbers). Its influence on a chemical reaction is subtle and difficult to detect.
This breakthrough serves as a typical example for the study of chemical reaction dynamics under the influence of spin-orbit interaction. A perspective article written by Prof. T. Peter Rakitzis on this work was also published on Science, titled “Transition states and spin-orbit structure.”
Prof. Xueming Yang, Prof. Zhigang Sun, and Prof. Xing’an Wang are the corresponding authors of the paper. This work was supported by the National Key Research and Development Program of China and the National Natural Science Foundation of China.
Paper link: https://science.sciencemag.org/content/371/6532/936
Perspective article: https://science.sciencemag.org/content/371/6532/886




