SUSTech Chuang-Chuang Li’s group achieves first and asymmetric total synthesis of phomarol
Chuangchuang LI 2021-03-18
Recently, Professor Chuang-Chaung Li’s team from the Department of Chemistry at the Southern University of Science and Technology (SUSTech) published their recent research paper in the academic journal CCS Chemistry, entitled “Asymmetric Total Synthesis of Phomarol”.
Phomarol (1) (Figure 1), a C25 steroid with an unusual framework, was isolated in 2016 from a jellyfish-derived fungus. Structurally, phomarol has a sterically compact [7–6–6–5–6]-fused pentacyclic architecture, with a highly functionalized tetrahydropyran E-ring (highlighted in blue). Furthermore, phomarol bears a synthetically challenging C12 oxidation, for which there are limited efficient approaches to install. Phomarol has eight stereocenters, including two quaternary centers. In addition, phomarol possesses a benzocycloheptane A/B ring system (highlighted in red), which is unprecedented in steroids. Therefore, phomarol presents a significant synthetic challenge.
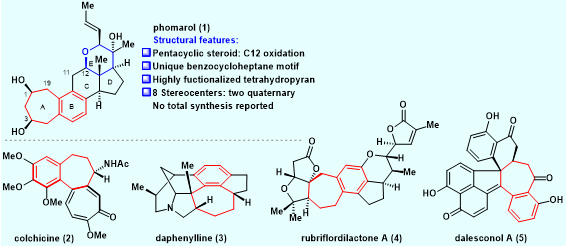 Figure 1. Structural features of phomarol and selected natural products containing a benzocycloheptane motif (highlighted in red)
Figure 1. Structural features of phomarol and selected natural products containing a benzocycloheptane motif (highlighted in red)
Professor Chuang-Chaung Li’s group is very interested in the total synthesis of natural products with the benzocycloheptane motif. In 2017, they achieved the concise total synthesis of natural drug colchicine only in a total of nine steps using intramolecular [5 2] cycloaddition as a key reaction, and a new lead compound with anti-tumor bioactivity was found in this study. Recently, a report from the same group entitled “Recent advances in total syntheses of natural products containing the benzocycloheptane motif” was published online in Natural Product Reports (NPR), a top review journal in the field of natural products. In this review, recent progress (2010-2020) in the total syntheses of natural products bearing the benzocycloheptane motif is presented, and key transformations for the construction of benzocycloheptane are highlighted. They also discuss the future development in this important research area.
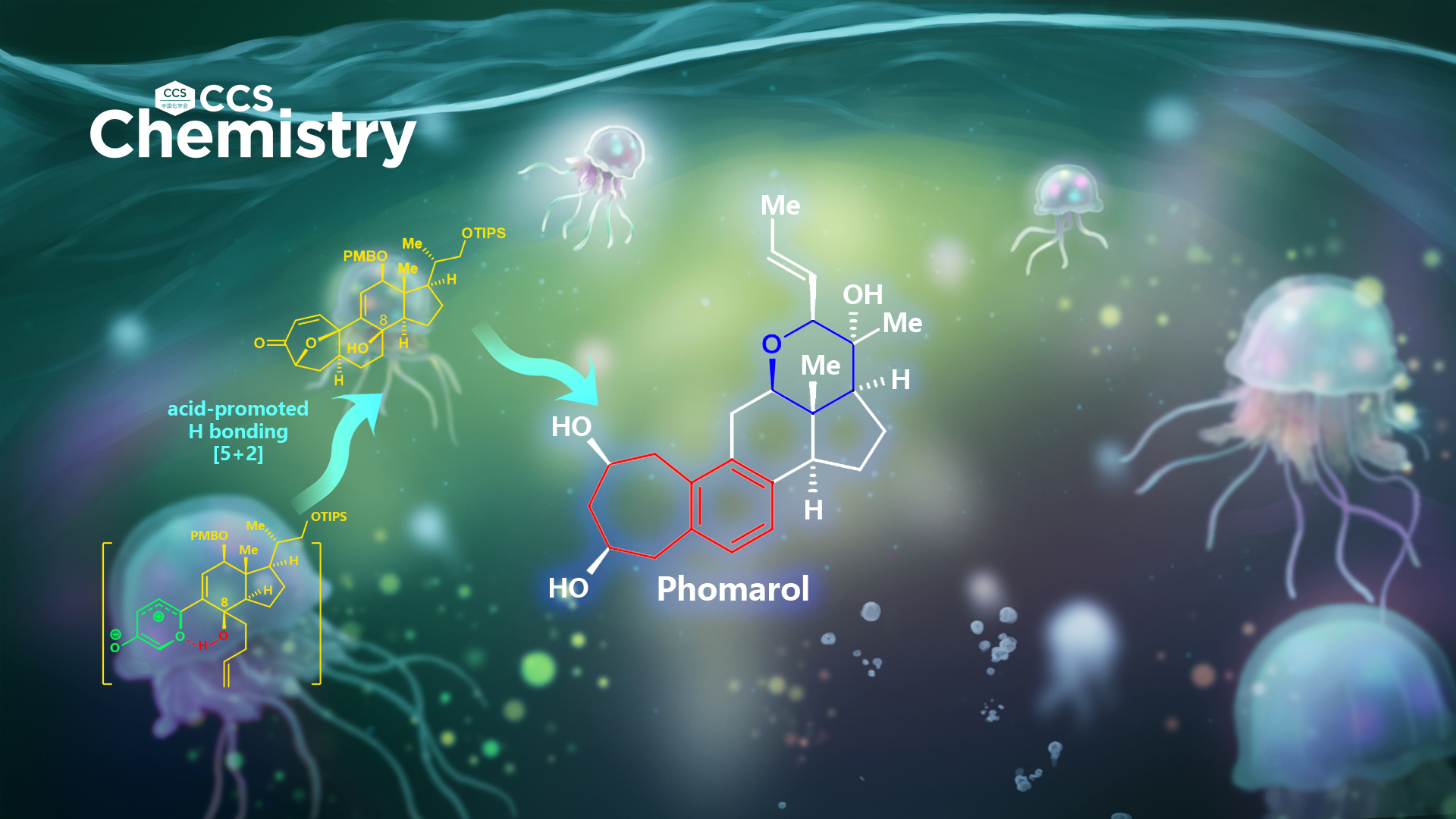
After an extensive investigation over several years, Li’s group has achieved the first and asymmetric total synthesis of phomarol, definitively establishing the absolute configuration (Figure 2). Notably, the synthetically challenging benzocycloheptane motif, which is also found in other natural products (Figure 1) and medicines, was synthesized efficiently using a very mild type I [5 2] cycloaddition promoted by acid, followed by regio- and chemoselective cleavage of the C–O bond and aromatization cascade.
This work represents the first example of an acid-promoted [5 2] cycloaddition applied successfully in the total synthesis of natural products. It is also the first example of using hydrogen bonding between the hydroxy group and oxidopyrylium ylide to control the stereoselectivity of cycloadditions. Furthermore, the challenging and highly functionalized tetrahydropyran ring of phomarol was successfully installed based on their suggested biomimetic pathway. This method could be extended to the total synthesis of other biologically active natural products containing the benzocycloheptane motif, to enable further biological investigation.
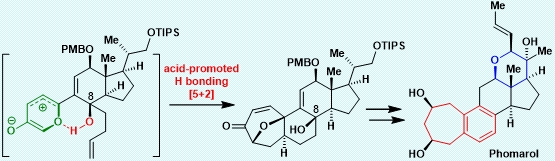 Figure 2. The first and asymmetric total synthesis of phomarol
Figure 2. The first and asymmetric total synthesis of phomarol
In conclusion, the first and asymmetric total synthesis of phomarol, an uncommon C25 steroid, was achieved. The synthetically challenging benzocycloheptane motif in phomarol was synthesized efficiently using a very mild acid-promoted type I [5 2] cycloaddition, followed by regio- and chemoselective cleavage of the C–O bond and aromatization cascade. The highly functionalized tetrahydropyran ring of phomarol was produced efficiently based on their suggested biomimetic pathway.
Dr. Jian-Hong Fan from the Department of Chemistry at SUSTech is the first author. Professor Chuang-Chuang Li from the Department of Chemistry at SUSTech is the corresponding author. This research was supported by postgraduate student Jing-Jing Wang and Associate Professor Lung Wa Chung from the Department of Chemistry at SUSTech.
This work was supported by the Natural Science Foundation of China, the Shenzhen Science and Technology Innovation Committee, and the Shenzhen Peacock Plan Project.
Paper link: https://www.chinesechemsoc.org/doi/10.31635/ccschem.021.202000721
Previous NPR paper link: https://pubs.rsc.org/en/content/articlepdf/2021/NP/D1NP00003A