Achieving asymmetric total synthesis of Taxol
Ya-Jian HU 2021-10-20
Taxol (Figure 1) is one of the most famous natural diterpenoids and important anticancer medicine. Structurally, Taxol has a highly oxygenated [6-8-6-4] core with eleven stereocenters and a bridgehead double bond. The extremely strained bicyclo[5.3.1] undecene ring system in Taxol is a unique bridged skeleton. Hence, Taxol represents a formidable synthetic challenge and has prompted significant interest from the synthetic community (>40 groups from all over the world) due to its fascinating architectural features and excellent antitumor activity.
However, in all the previous syntheses of Taxol, there have been no reports of closing the desired eight-membered ring through C1-C2 bond formation. Furthermore, the existence of Taxol-resistant tumors and side effects of Taxol makes the development of new approaches to synthesize Taxol and its derivatives highly desirable.
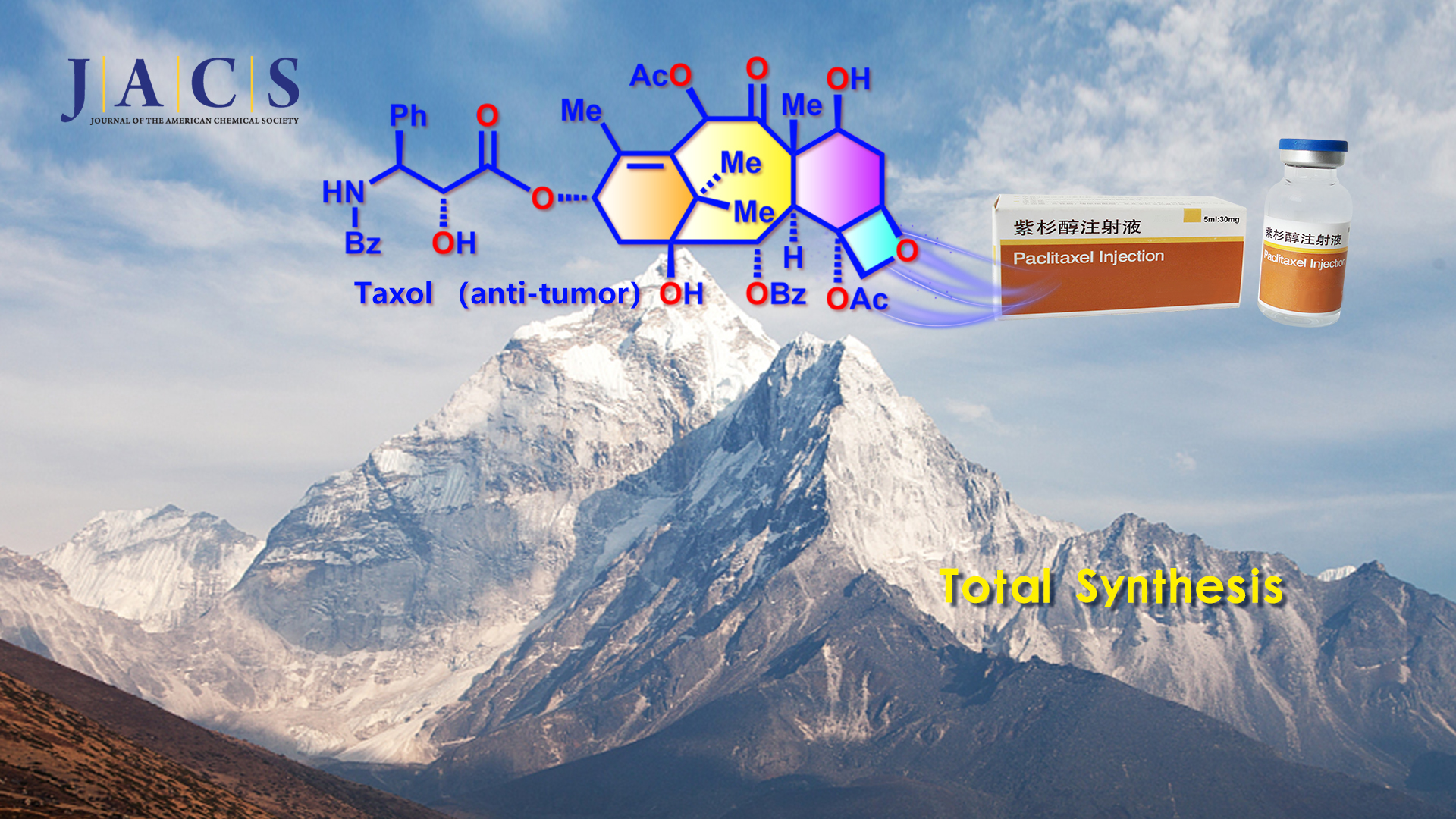
Recently, Professor Chuang-Chuang Li’s team from the Department of Chemistry at the Southern University of Science and Technology (SUSTech) completed an asymmetric total synthesis of Taxol using a concise approach through 19 isolated intermediates. The challenging eight-membered ring was constructed efficiently by a diastereoselective intramolecular SmI2-mediated pinacol coupling reaction to form the C1−C2 bond.
The unique biomimetic oxygen ene reaction and the newly developed facile tandem C2-benzoate formation and C13 side chain installation improved the synthesis efficiency. The mild oxygen ene reaction under light conditions would be an alternative reaction involved in Taxol biosynthesis.
Their study, entitled “Asymmetric Total Synthesis of Taxol,” was published in the Journal of the American Chemical Society, a high-impact journal that is considered one of the most impactful and prestigious journals in chemistry, on October 12, 2021.
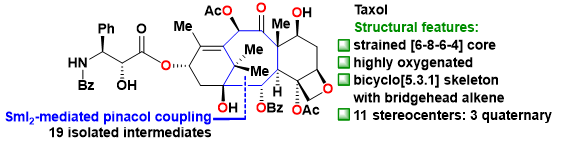 Figure 1. Structural features of Taxol
Figure 1. Structural features of Taxol
Prof. Chuang-Chuang Li’s group completed the total asymmetric synthesis of Taxol using a concise approach through 19 isolated intermediates. Their work represents the shortest route to Taxol. The synthetically challenging eight-membered ring of Taxol was constructed efficiently via a diastereoselective intramolecular SmI2-mediated pinacol coupling to form the C1−C2 bond. The reported new convergent approach will allow the diverse creation of Taxol derivatives to enable further biological research and benefit the further development of Taxol-based antitumor drugs.
Dr. Ya-Jian Hu, master’s student Chen-Chem Gu, and Research Assistant Xin-Feng Wang, all members of the Department of Chemistry at SUSTech, are the co-first authors of this paper. Prof. Chuang-Chuang Li is the only corresponding author of this paper. Research Associate Professor Dr. Long Min from the Department of Chemistry at SUSTech also made significant contributions to this study.
This work was supported by the National Natural Science Foundation of China (NSFC).
Paper link: https://pubs.acs.org/doi/full/10.1021/jacs.1c09637




