Researchers make series of advancements in the field of photoelectric conversion and electrocatalysis
Zongxiang XU 2022-02-11
Associate Professor Zongxiang Xu’s team from the Department of Chemistry at the Southern University of Science and Technology (SUSTech) has made a series of research developments in the field of photoelectric conversion and electrocatalysis. They published four academic papers in renowned journals such as the Journal of the American Chemical Society, Angewandte Chemie, Journal of Energy Chemistry, and Chemical Engineering Journal.
Chiral organometallic light-emitting materials can emit left- and right-handed circularly polarized light (CPL), respectively, and have potential applications in circularly polarized organic light-emitting devices (CP-OLEDs) and 3D displays. However, since a two-dimensional planar complex molecule always overlaps its mirror image, it has always been a big challenge to use coordination to create chirality for planar complexes.
Prof. Xu’s team collaborated with Haifeng Xiang’s research group from the School of Chemistry at Sichuan University (SCU) to report binuclear Pt(II) complexes to construct metal coordination-induced face chirality. They firstly proposed the naming method of this kind of new chirality and resolved the problem of chiral separation using binaphthalene chiral induction. These complexes have high phosphorescence quantum yield (83.4%), and the as-fabricated high-efficiency CP-OLED achieves an external quantum efficiency of 14.3% and an asymmetry factor of 2.0×10-3 (Fig. 1).
Their research results, entitled “Highly Phosphorescent Planar Chirality by Bridging Two Square-Planar Platinum(II) Complexes: Chirality Induction and Circularly Polarized Luminescence,” were published in the Journal of the American Chemical Society.
Jin Song, a graduate student at SCU, and Hui Xiao, a postdoctoral fellow at SUSTech, are the co-first authors of this paper. Prof. Haifeng Xiang and Prof. Cheng Yang from SCU and Prof. Zongxiang Xu from SUSTech are the co-corresponding authors.
 Figure 1. Molecular design of binuclear Pt(II) complexes and CP-OLED devices
Figure 1. Molecular design of binuclear Pt(II) complexes and CP-OLED devices
Perovskite solar cells (PSCs) are among the most promising next-generation photovoltaic technologies for commercialization. Efficiency and stability has always been research hotspots in this field. Grain boundary defects, defects at heterojunction interfaces, and energy band offsets in perovskite thin films are considered to determine the power loss of PSCs during operation. At the same time, it affects their operational stability, which has become the main obstacle to the further practical application of PSCs.
The reported tuning strategies cannot simultaneously passivate the defects located at the grain boundaries of the perovskite bulk phase and the interface of the heterojunction. Comprehensively optimizing the heterojunction interface and grain boundary defect density, while regulating the energy level arrangement of the two adjacent interfaces of perovskite, has gradually become an effective strategy for realizing high-efficiency and high-stable PSC devices.
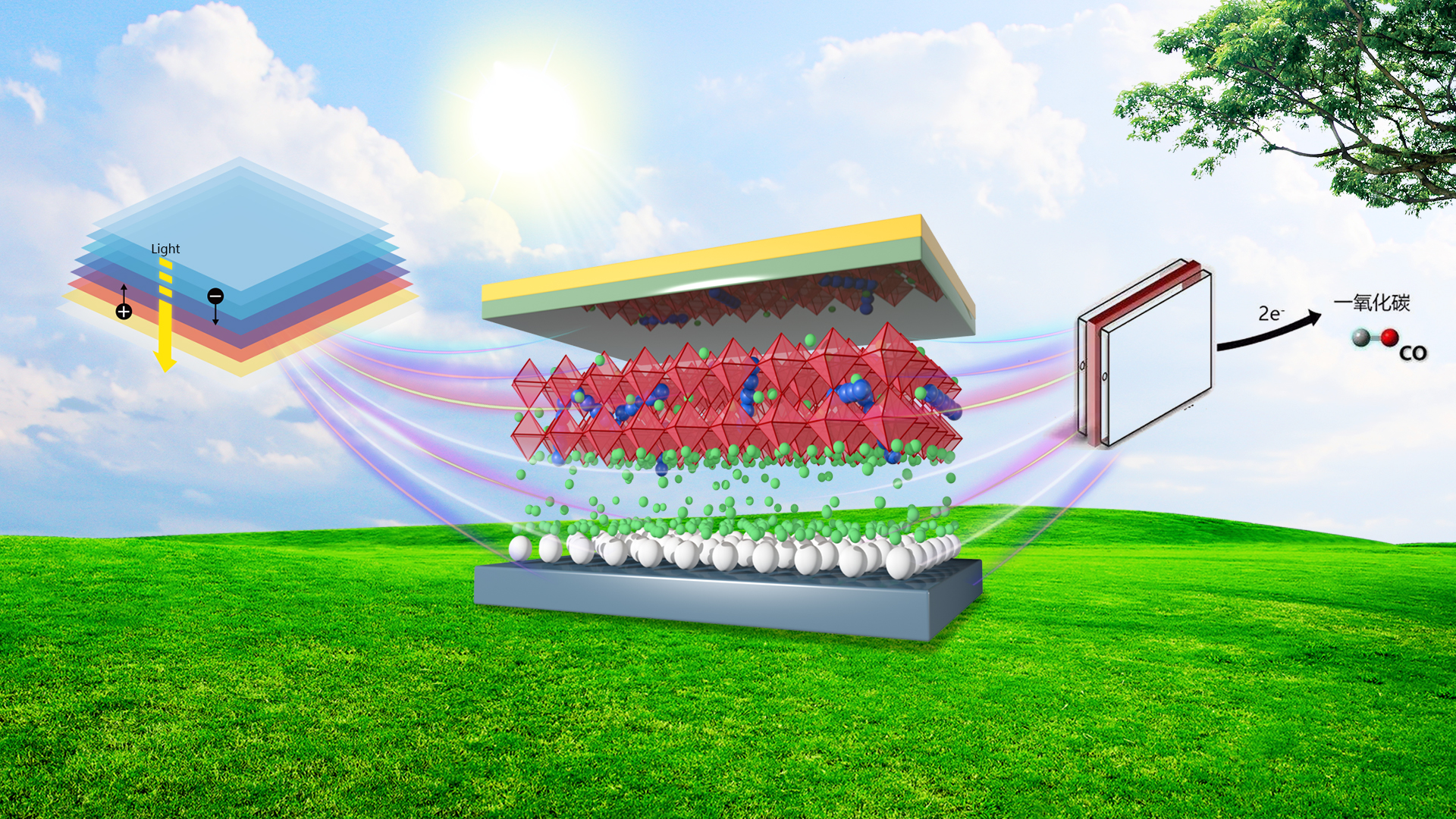
Prof. Xu’s group and Taiho Park of Pohang University of Science and Technology (POSTECH), South Korea, developed a quinacridone (QA) derivative, an amphiphilic π-conjugated ion compound (QAPyBF4) (Fig. 2) and used as a perovskite modulator. Due to the spontaneous diffusion and synergy effect of amphiphilic QAPyBF4, a dimensional modification strategy has been devised to bridge ETL-to-HTL contact throughout the active layer, improving band alignment at active interfaces, and reducing recombination losses.
The universal one-step additive process achieves multiple functions. Enriched [BF4]− and a certain amount of cations form interfacial dipoles and decrease detrimental defects, resulting in favorable energy level alignment at the ETL/perovskite interface and yielding barrier-free charge transport. P-type conjugated cations adsorbed on the perovskite surface with under-coordinated Pb cations decrease the WF of perovskite, forming ohmic contact between the perovskite and HTL layer, thereby boosting hole extraction at the grain boundaries and interface. Lastly, the ion synergistic effect forms a stabilized device configuration of SnO2/[BF4]–/perovskite:[QAPy]2 /HTL at the back contact via multiple chemical bonding, effectively inhibiting nonradiative recombination and reducing mass loss during device operation. Therefore, a signature VOC of 1.2 V and PCE of up to 23.1% can be achieved in the champion device with significantly enhanced stability.
These research results, entitled “Synergy Effect of a π-Conjugated Ionic Compound: Dual Interfacial Energy Level Regulation and Passivation to Promote VOC and Stability of Planar Perovskite Solar Cells,” were published in Angewandte Chemie.
Dr. Xiaoyuan Liu, a visiting scholar in Prof. Zongxiang Xu’s research group, is the first author of this paper. Prof. Xu is the co-corresponding author, and SUSTech is the first correspondence unit of the paper.
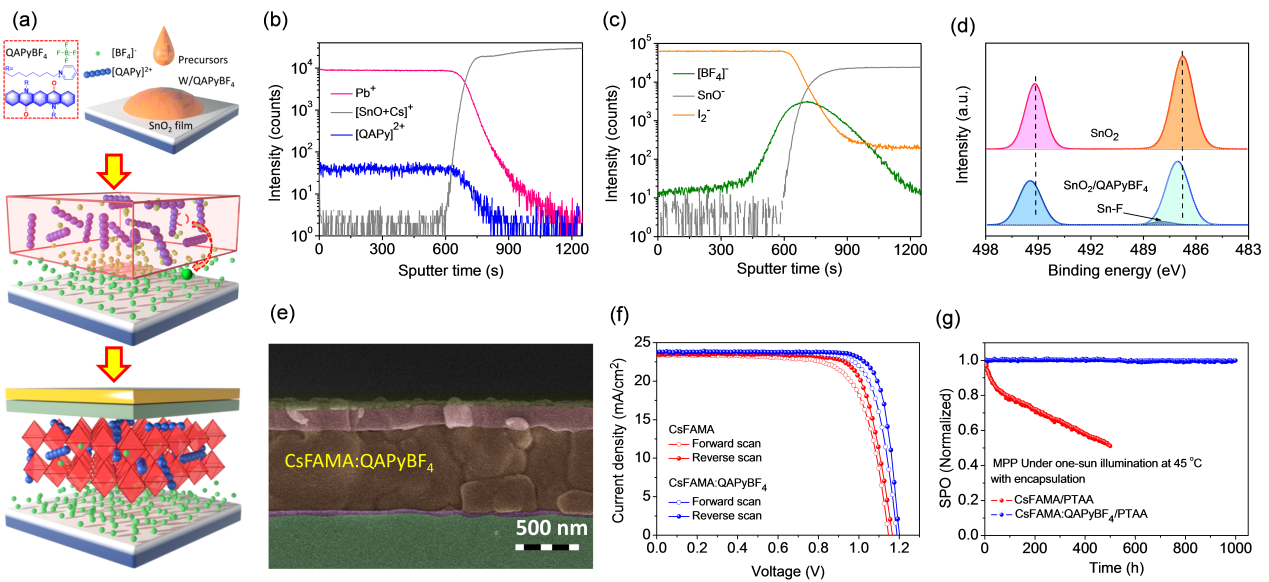 Figure 2. Preparation of perovskite devices; study on passivation mechanism and device performance
Figure 2. Preparation of perovskite devices; study on passivation mechanism and device performance
Prof. Xu’s research group has been conducting research on phthalocyanine molecular design and application in the field of optoelectronics for a long time. Their paper, entitled “Reformation of thiophene-functionalized phthalocyanine isomers for defect passivation to achieve stable and efficient perovskite solar cells,” was published in the Journal of Energy Chemistry. It introduces the use of Lewis acid-base passivation strategy to overcome the problems of perovskite grain boundary width, crystal defects, and grain boundary instability with achieving an improved performance of perovskite solar cells.
In this study, the research group cooperated with Dr. Ilgın Nar of Istanbul Technical University (ITU), Turkey, to design and synthesize thiophene-modified phthalocyanine isomers (S2 and S3) as Lewis base passivators for perovskite preparation (Fig. 3). Through density functional theory calculations, it was confirmed that the position of the S atom in the phthalocyanine structure affects the interaction with the undercoordinated Pb2 site defects in the perovskite.
S3 can better passivate the perovskite, resulting in improved morphology, larger film grain size, and denser surface, leading to improved charge extraction capability and reduced nonradiative recombination and defect density. The highest power conversion efficiency of the S3 passivated device reaches 18%, which is 6.69% higher than that of the reference device. The device exhibits remarkable stability under high temperatures and high humidity.
Geping Qu, a graduate student in Prof. Zongxiang Xu’s research group, and Danish Khan, postdoctoral fellow, are the co-first authors of this paper. Prof. Xu is the co-corresponding author, and SUSTech is the first unit of the paper.
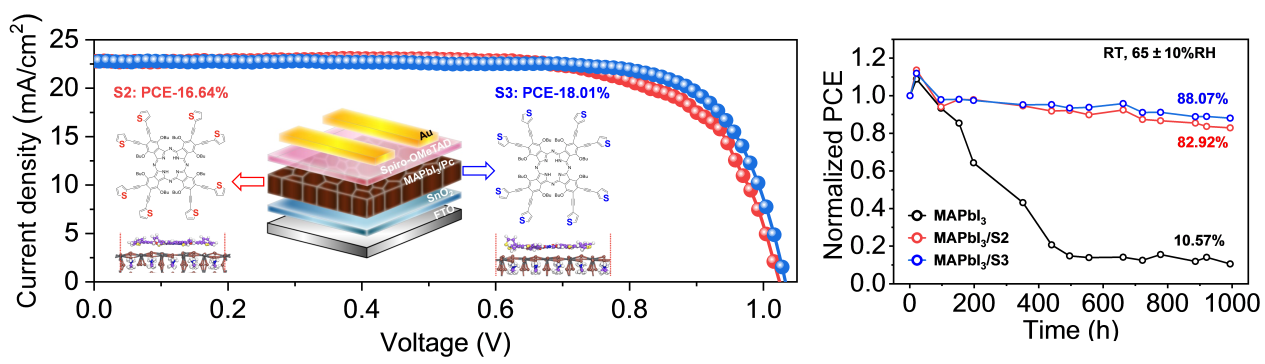 Figure 3. Molecular structure of isomeric phthalocyanine passivators and performance of PSC devices
Figure 3. Molecular structure of isomeric phthalocyanine passivators and performance of PSC devices
Electrochemical CO2 reduction reaction (CO2RR) is one of the most promising CO2 conversion methods, which can convert CO2 into valuable chemicals (such as CO, CH4, HCOOH, CH3OH, etc.) to mitigate global warming. Therefore, it is crucial to develop electrocatalysts with efficient CO2RR.
Prof. Xu’s group used a facile precipitation method to synthesize a non-peripheral octamethyl-substituted Cobalt(II) phthalocyanine complex (N-CoMe2Pc) nanorod (Fig. 4). DFT calculations indicate that the N-CoMe2Pc catalyst exhibits enhanced CO2 adsorption and activation on the Co surface at low overpotentials compared with pristine Cobalt(II) phthalocyanine (CoPc). Because of the significantly increased electron density, the number of exposed active sites, and electrical conductivity induced by the nanostructure and eight methyl groups, the N-CoMe2Pc nanorods exhibited higher CO2RR activity. The charge transfer kinetics of N-CoMe2Pc was further improved by immobilization on nitrogen-doped reduced graphene oxide (NRGO), which achieved a higher FECO (94.1%) under CO current density of 56.4 mA cm-2 in alkaline solution. A good turnover frequency (TOFCO = 6.2 s-1) at lower potentials (-0.6 V vs. RHE) was achieved.
Additionally, the flow cell and alkaline electrolyte device facilitated the CO2RR activity and selectivity of the N-CoMe2Pc/NRGO nanocomposite, facilitating its mass transfer and significantly suppressing the HER reaction, as well as better alkaline electrolyte conductivity.
These research results, entitled “Non-peripheral octamethyl-substituted Cobalt phthalocyanine nanorods supported on N-doped reduced graphene oxide achieve efficient electrocatalytic CO2 reduction to CO,” were published in Chemical Engineering Journal.
Minzhang Li, a graduate student in Prof. Zongxiang Xu’s research group, is the first author of this paper. Prof. Xu is the sole corresponding author.
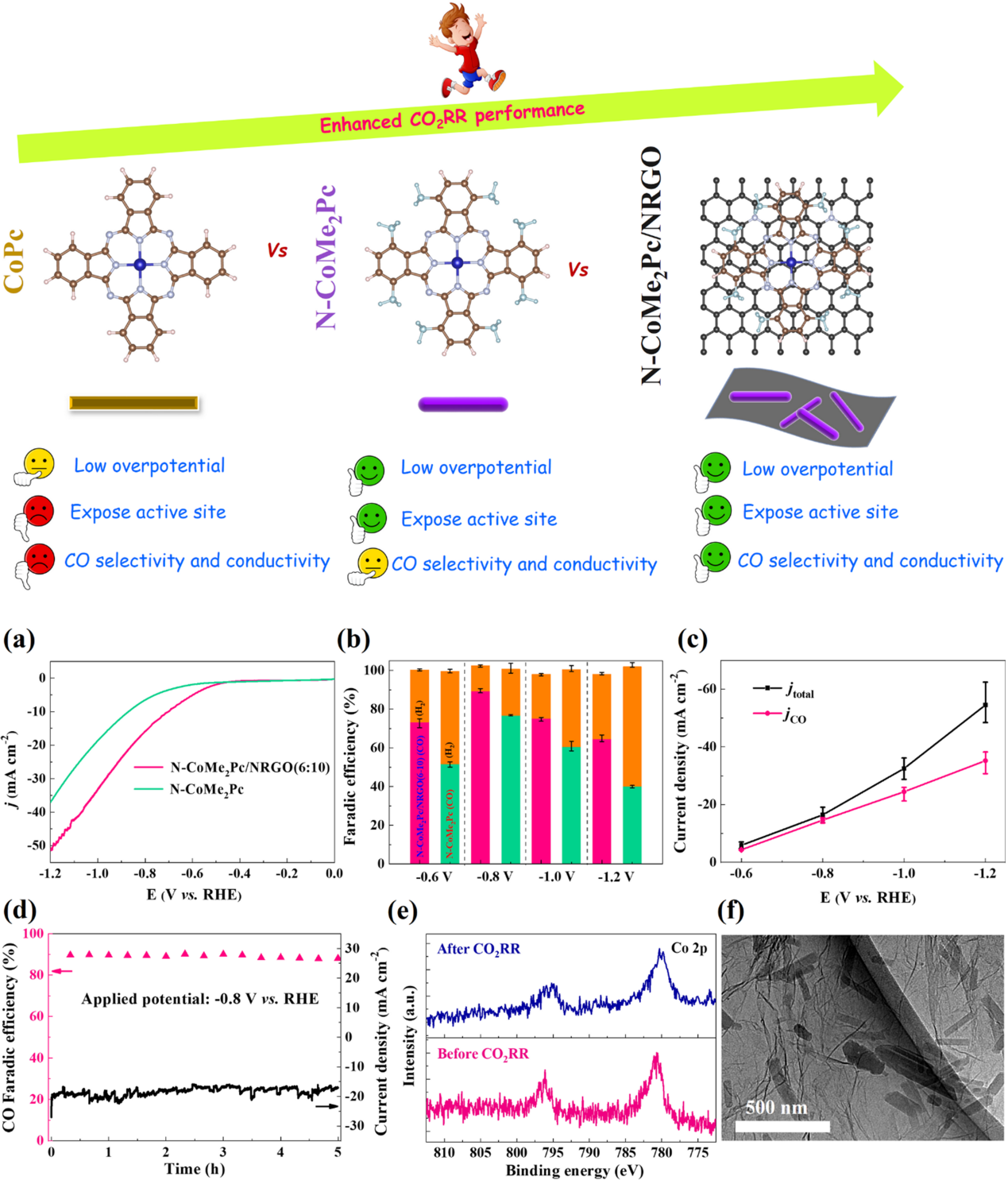 Figure 4. Phthalocyanine catalyst structure and CO2 electrocatalytic reduction performance
Figure 4. Phthalocyanine catalyst structure and CO2 electrocatalytic reduction performance
The above studies have been funded by the National Natural Science Foundation of China (NSFC), the Guangdong-Hong Kong-Macao Joint Laboratory for Photonic-Thermal-Electrical Energy Materials and Devices, and the Major Program of Guangdong Basic and Applied Research from the Guangdong Provincial Department of Science and Technology.
The researchers would also like to acknowledge the strong support from Prof. Hu Xu of the Department of Physics, Associate Professor Fei Wang of the School of Microelectronics, Associate Professor Lele Duan of the Department of Chemistry, and the Public Analysis and Testing Center of SUSTech.
Paper links (In order of appearance above):
Journal of the American Chemical Society: https://doi.org/10.1021/jacs.1c11699
Angewandte Chemie: https://doi.org/10.1002/ange.202117303
Journal of Energy Chemistry: https://doi.org/10.1016/j.jechem.2021.09.041
Chemical Engineering Journal: https://doi.org/10.1016/j.cej.2021.133050




