Breaking into the black box of molecular self-assembly – A case study of stepwise construction of high-nuclearity lanthanide clusters
2022-06-21
It is fundamentally significant to be able to construct sophisticated molecular architectures with precise structural control when we seek molecule-based materials with specific properties and functions. However, the obtainment of such complex entities has mostly been serendipitous. Their syntheses remain more of an art than a science and are frequently characterized by random self-organization.
The research team led by Chair Professor Zhiping Zheng of the Department of Chemistry at the Southern University of Science and Technology (SUSTech) has recently made significant progress toward addressing this general inability to rationally create the target molecules of interest.
Their research, entitled “Anion-Guided Stepwise Assembly of High-Nuclearity Lanthanide Hydroxide Clusters”, was selected as a VIP (Very Important Paper) and published online in Angewandte Chemie International Edition, one of the prime chemistry journals in the world today.
The researchers reported a rare example of modular assembly of otherwise synthetically elusive high-nuclearity lanthanide clusters guided by anion templates that are judiciously selected and step wisely introduced (Figure 1). These structurally appealing polynuclear cluster species are of interest for optical, magnetic, and catalytic applications.
Prof. Zheng’s team succeeded in the anion-directed stepwise assembly of the cage-like cluster complex featuring a 60-metal core (Er60). The whole synthetic journey is summarized in Figure 1. In Step I, based on their previous studies on the halogen template effect (Inorg. Chem. Front. 2021, 8, 26.), I− ions were used as templates to control the assembly of a tetragonal wheel-like Er12 – one of the two essential building motifs of the complete cage of Er60 (Figure 1, yellow part).
In Step II, CO32− ions were introduced as the second template to direct the formation of two hexagonal units (Figure 1, red part) that flank the opposite sides of the tetragonal Er12, producing a boat-like Er34. In Step III, NO3− ions were added to further expand the growth of the cluster core, constructing one newly grown hexagonal unit (Figure 1, turquoise part) in a bowl-like Er48. Finally, the cage-like Er60 was achieved under hydrothermal conditions with the closing-up of the cage from Er48, completing the stepwise assembly with the isolation and structural identification of the series of cluster intermediates.
It is of particular note that the three cluster intermediates, corresponding respectively to 1/6, 1/2, and 3/4 of the complete cage of Er60, not only leave a trace of the reaction path, but also constitute a closely related and gradually evolving set of intermediates to complete the “jigsaw puzzle” of the cluster assembly while gaining the all-valuable mechanistic insights.
The present rational synthesis stands in stark contrast to the “mix and pray” practice as sometimes dubbed by critics. It represents possibly the very first piece of effort to open the “black box” of lanthanide cluster assembly by deciphering the “code” along the modular and stepwise assembly pathway. This work is expected to stimulate further efforts for the directed synthesis of complex molecules with highly sophisticated structures and possibly useful properties.
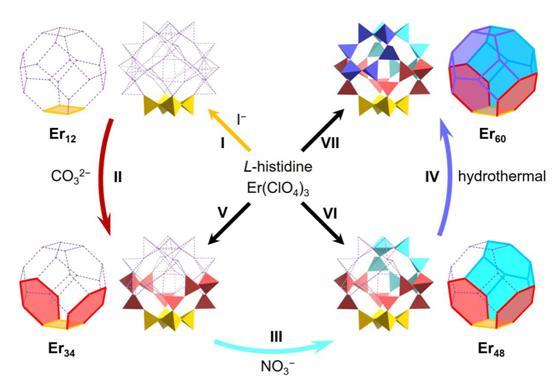
Figure 1. Anion-guided stepwise assembly of a high-nuclearity lanthanide hydroxide cluster Er60 with the identification of key intermediate clusters Er12, Er34, and Er48. Each vertex of the truncated octahedron represents a cubane-like [Er4(OH)4] unit.
Graduate student Weiming Huang is the first author of this paper. Research Asst. Prof. Min Feng and Prof. Zhiping Zheng are the co-corresponding authors.
This work was financially supported by the National Natural Science Foundation of China (NSFC), the Stable Support Plan Program of Shenzhen Natural Science Fund, and the SUSTech Startup Fund.
Paper link: https://doi.org/10.1002/anie.202205385




