Researchers make breakthrough in enantioconvergent N-alkylation of aliphatic amines
2023-03-28
Chiral amines are the most common functional groups for stereochemically complex molecules in the pharmaceutical and agrochemical industries. The strong demand for unnatural chiral amines has driven numerous racemic amine synthetic methods to become catalytically asymmetric.
N-alkylation of amines with alkyl halides was discovered by Hofmann in 1850, which was the most straightforward and well-adopted method to construct high-value-add amines from readily available starting materials. However, the asymmetric version of this reaction has thus far largely relied on the use of enantioenriched alkyl halides, of which the stereoselective synthesis may be arduous.
Major obstacles in this aspect include the high Lewis basicity of aliphatic amines and ammonia that likely leads to transition metal catalyst poisoning, and the selective deprotonation of the weakly acidic N–H bonds of these amines without non-stereoselective N-alkylation in the presence of alkyl halides.
Chair Professor Xin-Yuan Liu’s research team from the Department of Chemistry at the Southern University of Science and Technology (SUSTech) has recently made a breakthrough in the enantioconvergent N-alkylation of aliphatic amines to meet the strong synthetic demand for constructing chiral aliphatic amines in both academic research and pharmaceutical industries.
Their research, entitled “Enantioconvergent Cu-catalyzed N-alkylation of aliphatic amines”, has been published in Nature.
To overcome the aforementioned difficulties and given the great application potential, the researchers envisaged that finely tuned chiral multidentate anionic ligands with strong binding affinities to metal catalysts would not only overcome the catalyst poisoning by aliphatic amines or ammonia but also elicit high chemo- and enantioselectivity. The reaction successfully accommodates a diverse range of aliphatic amines (>100 examples) that are readily available industrial feedstocks, drug molecules, or building blocks and even tolerates, although partially, the most challenging ammonia.
Excellent enantioselectivity and functional group tolerance were observed. This method can directly convert feedstock chemicals including ammonia and pharmaceutically-relevant amines into unnatural chiral α-amino amides derivatives under mild conditions. The power of the transformation has been demonstrated in a number of complex settings, including achieving late-stage functionalization and expedited synthesis of diversely complex amine drug molecules with high catalyst-controlled stereoselectivity. Mechanism studies indicated that chiral multidentate anionic ligands are crucial solution for overcoming transition metal catalyst poisoning and enantioselective controlling.
This strategy would enable a general catalytic platform for constructing chiral amines and will be widely adopted by the pharmaceutical industry for its excellent heteroatomic nucleophile tolerance.
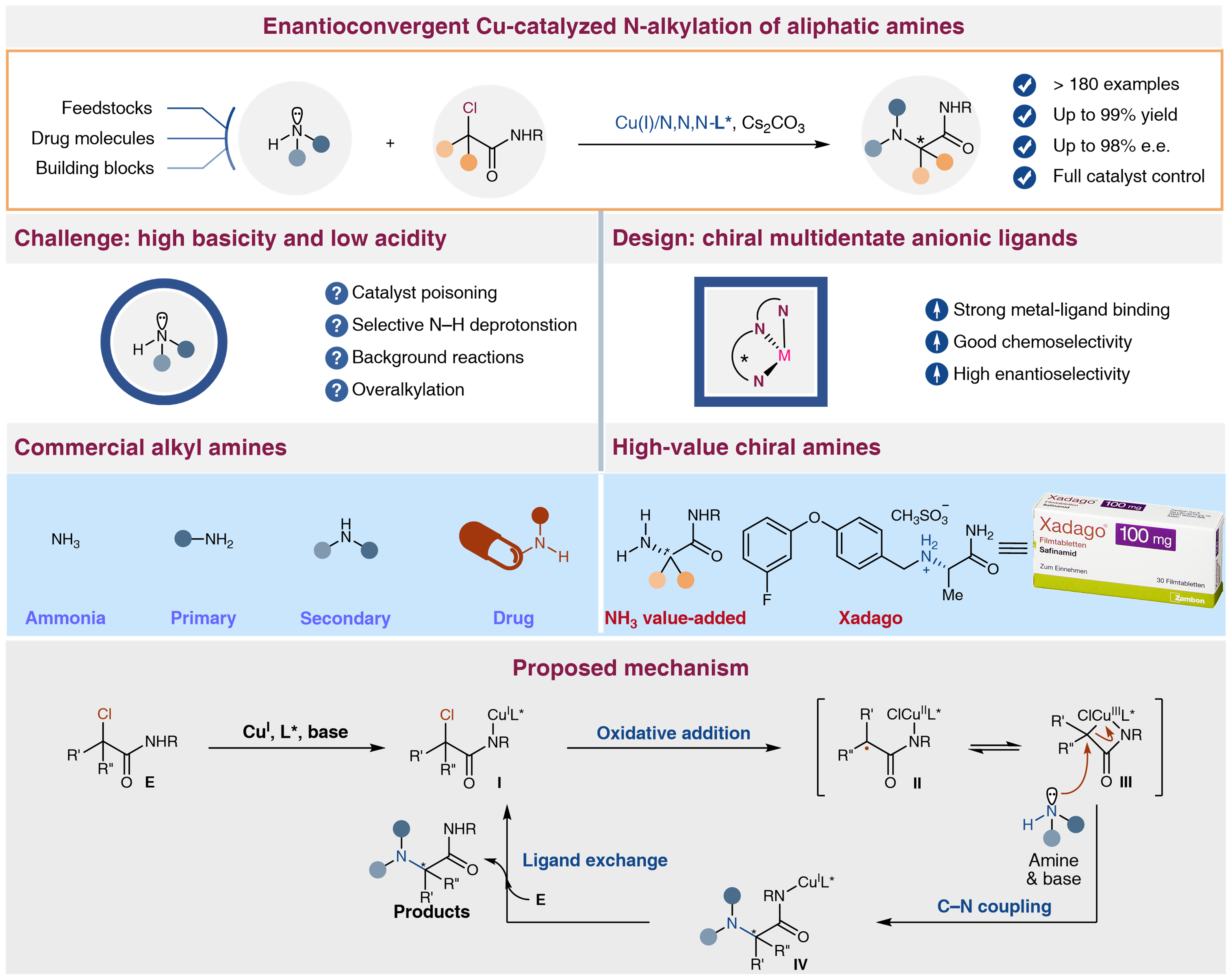
Figure 1. Enantioconvergent Cu-catalyzed N-alkylation of aliphatic amines
Research Asst. Prof Ji-Jun Chen, along with Ph.D. students Jia-Heng Fang and Xuan-Yi Du, all from the Department of Chemistry at SUSTech, are the co-first authors of this paper. Chair Professor Xin-Yuan Liu is the corresponding author, and SUSTech is the corresponding unit of the paper.
Other co-authors from SUSTech included Zhe Dong, Qiang-Shuai Gu, Zhong-Liang Li, Jia-Yong Zhang, Jun-Qian Bian, Fu-Li Wang, Cheng Luan, Wei-Long Liu, Ji-Ren Liu, and Xiao-Yang Dong.
This work was supported by the National Key R&D Program of China, National Natural Science Foundation of China (NSFC), Guangdong Innovative Program, Shenzhen Special Funds, and the SUSTech Special Fund for the Construction of High-Level Universities.
Paper link: https://www.nature.com/articles/s41586-023-05950-8




