Researchers unlock new frontiers in carbene chemistry
2024-01-16
Carbene chemistry stands at the forefront of molecular exploration, offering a captivating journey into the unique behavior of carbon species that challenge conventional rules. Carbenes, compounds containing a divalent carbon atom with only six electrons in its valence shell, are very intriguing to scientists.
While most carbon compounds play it safe, carbenes become unique and very reactive, making them useful in various areas like creating materials and medicines. Understanding how carbenes work and discovering new types opens up fresh possibilities for developing materials and advancing science.

A research group led by Associate Professor Liu Leo Liu from the Department of Chemistry at the Southern University of Science and Technology (SUSTech) has recently made progress in the field of carbene chemistry. Using a “coordination-fixation” strategy and the push-pull electronic effects of substituents, they successfully synthesized an ambiphilic carbene with a reverse electronic configuration (σ0π2 type). The isolation and characterization of these carbenes expand our understanding of carbon chemistry and hold the potential to transform the field of ambiphilic main group chemistry (Figure 1a).
Their research, entitled “A stable rhodium-coordinated carbene with a σ0π2 electronic configuration”, has been published in the prestigious international academic journal Science.
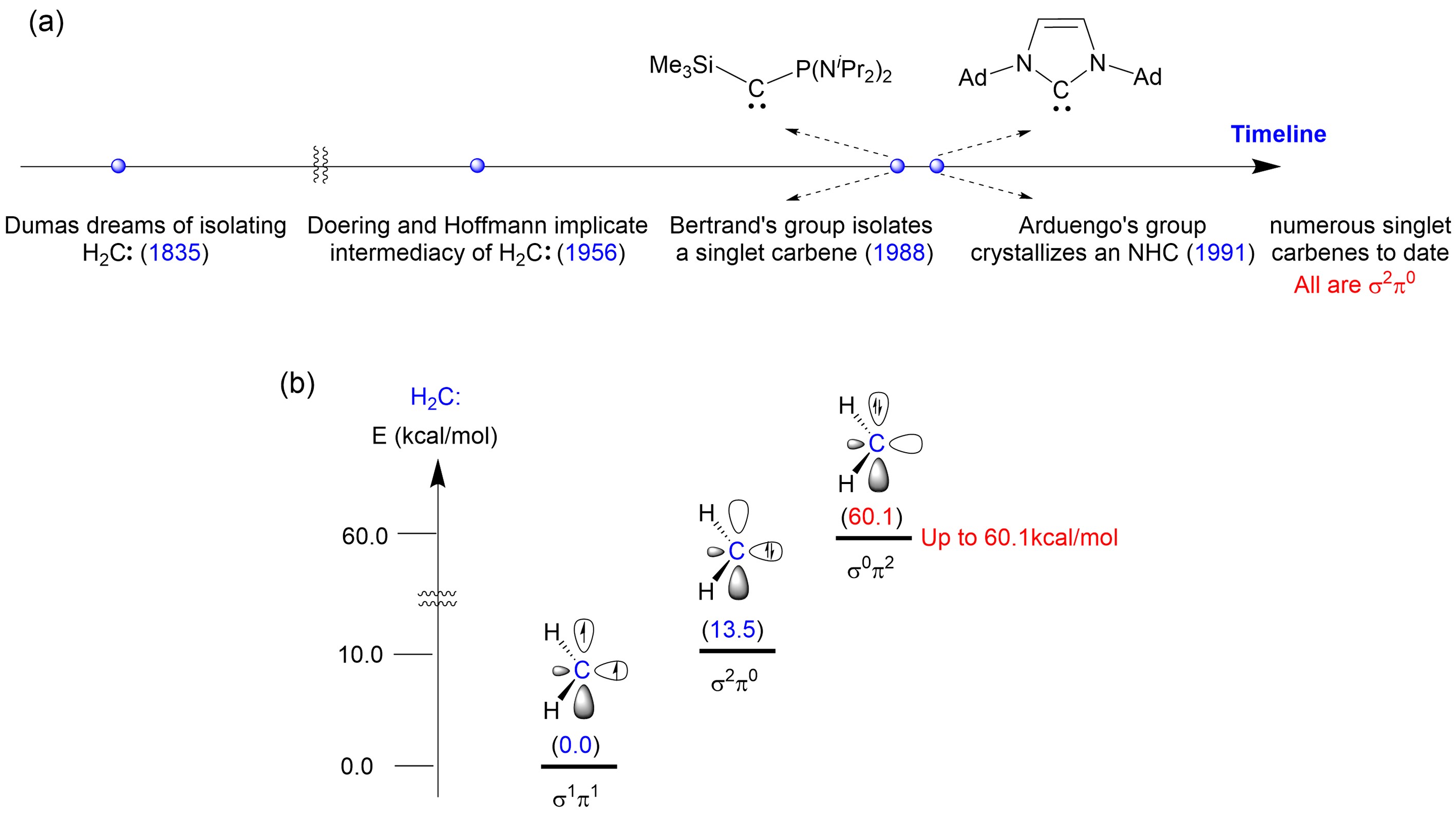
Figure 1. (a) Timeline of carbene chemistry development; (b) Energies of different electronic configurations of H2C: carbene. Image source: Science
In the realm of carbon chemistry, carbon atoms typically adhere to the octet rule. However, exceptions exist, such as carbon cations and carbon radicals. Carbenes (R2C:) represent another category of carbon species that defies the octet rule, possessing only six electrons in their valence shell, including two unpaired electrons. These highly reactive and versatile molecules have become important chemical tools, widely applied in catalysis, materials science, and pharmaceutical chemistry.
The parent carbene, H2C:, exhibits a ground state of triplet, with two single electrons occupying the in-plane σ orbital and out-of-plane π orbital of the carbene carbon, classifying it as a σ1π1 electronic configuration carbene. This configuration imparts its extremely high reactivity. Scientists have previously discovered that π-electron donating substituents can elevate the π orbital energy level of carbenes, allowing the stabilization and characterization of singlet carbenes with a σ2π0 configuration. However, filling both unpaired electrons of carbenes in the π orbital to construct a stable σ0π2 carbene has proven challenging and has been attempted by researchers worldwide for half a century without success (Figure 1b).
The research group led by Prof. Liu, focusing on ambiphilic main group chemistry, has pioneered innovative approaches in addressing the fundamental scientific question of how to stabilize ambiphilic structural units. Through rational design, the team has successfully isolated, characterized, or in-situ generated a series of ambiphilic main group compounds, deeply exploring the chemical properties and potential applications of these novel structural units.
In the field of main group chemistry, the researchers have made four critical scientific contributions: 1) creation of σ0π2 type ambiphilic carbene; 2) creation of tri-active ambiphilic carbyne anion (R−C−); 3) development of tri-active ambiphilic aluminylene (R−Al); 4) expansion of main group anions and multiply bonded compounds as precursors for generating of multi-active ambiphilic phosphorus/boron species. The group has published papers in Science, Nature Synthesis, Chem, Journal of the American Chemical Society, Angewandte Chemie International Edition, and CCS Chemistry. The research work has been highlighted or previewed by ChemistryViews, Nature Synthesis, and Chem.
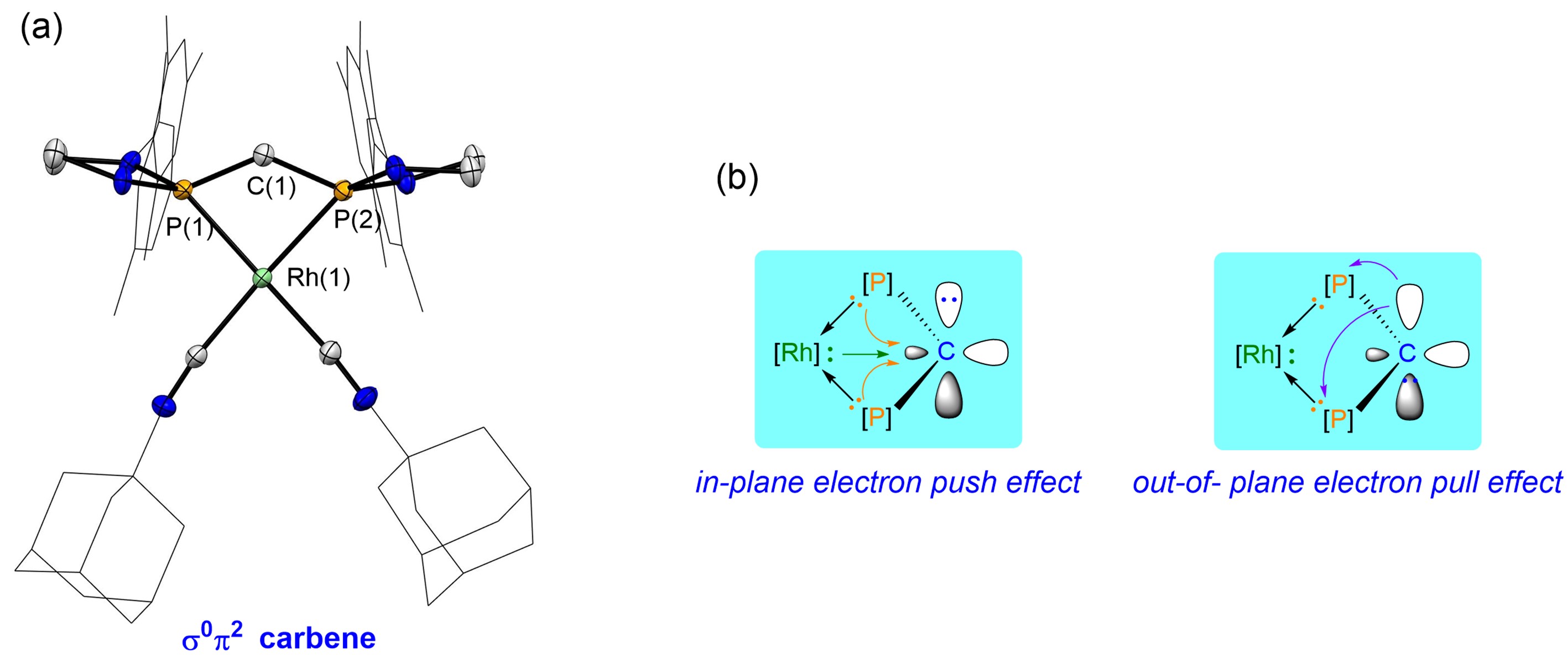 Figure 2. (a) Crystal structure of the σ0π2 Carbene; (b) Principle of stabilizing σ0π2 carbenes. Image source: Science
Figure 2. (a) Crystal structure of the σ0π2 Carbene; (b) Principle of stabilizing σ0π2 carbenes. Image source: Science
The researchers achieved a breakthrough by utilizing the “planar push electron effect” of substituents to elevate the σ orbital energy level of the carbene carbon. Simultaneously, employing the “out-of-plane pull electron effect” of substituents lowered the π orbital energy level of the carbene carbon. This resulted in the successful characterization of the first-ever ambiphilic carbene with a reverse electronic configuration (σ0π2 type), as depicted in Figure 2a. Nuclear Magnetic Resonance (NMR) spectra indicate a chemical shift of approximately -30 ppm for the carbene carbon, markedly different from the chemical shift of traditional singlet carbene carbon (usually greater than 180 ppm).
Through theoretical calculations, the study introduces a novel concept for stabilizing σ0π2 type carbenes. It clearly delineates the stabilizing mechanisms, which involve in-plane push electron effects and out-of-plane pull electron effects, a concept that is effectively illustrated in Figure 2b. Experimental studies verified the σ0π2 electronic configuration of the carbene, as shown in Figure 3. All compounds underwent characterization through NMR, crystallography, high-resolution mass spectrometry, and other techniques.
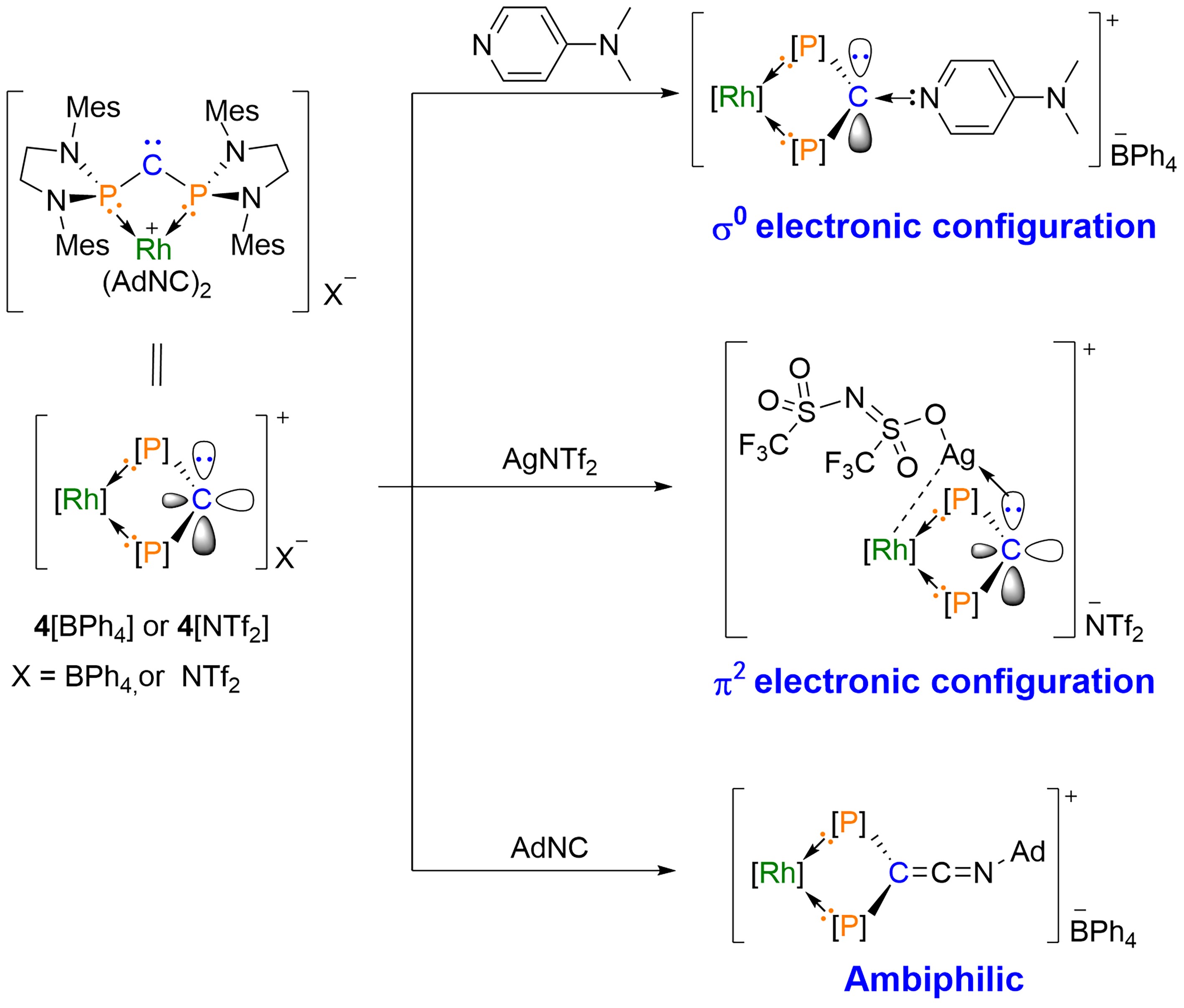 Figure 3. Reactivity of σ0π2 Carbenes; Mes = 2,4,6-Me3C6H2, Ad = Adamantyl; Image Source: Science
Figure 3. Reactivity of σ0π2 Carbenes; Mes = 2,4,6-Me3C6H2, Ad = Adamantyl; Image Source: Science
This research fills the gap in the characterization of stable σ0π2 carbenes, expanding our understanding of carbon chemistry. Due to their unique electronic properties, σ0π2 ambiphilic carbenes hold the potential to transform the field of ambiphilic main group chemistry.
Chaopeng Hu, a senior research fellow from the Department of Chemistry at SUSTech, is the first author of the paper. Associate Professor Liu Leo Liu is the sole corresponding author, and SUSTech is the sole corresponding unit. Doctoral student Xin-Feng Wang, senior research fellow Jiancheng Li, and Assistant Professor Xiao-Yong Chang from the Department of Chemistry also participated in this research.
This work was supported by the National Natural Science Foundation of China (NSFC), Shenzhen Science and Technology Innovation Program, Guangdong Innovation and Entrepreneurial Research Team Program, and the Guangdong Provincial Key Laboratory of Catalysis.
Paper link: https://www.science.org/doi/10.1126/science.adk6533