Enantioselective radical chemistry breakthrough paves way for new drug intermediates
2025-04-18
Radicals, characterized as highly reactive intermediates possessing unpaired single electrons, represent ubiquitous entities spanning from biological systems to cosmic scales. Since the structural confirmation of free radicals in 1900, radical chemistry has played a pivotal role in fields such as synthetic chemistry, biology, pharmacy, and materials science. However, the extreme reactivity of these species renders the achievement of reactive selectivity profoundly challenging.
The construction of chiral molecules with high enantioselectivity has emerged as a critical frontier in contemporary chemistry. Since the 1980s, chemists have successively pioneered multiple catalytic strategies—such as organocatalysis, transition-metal catalysis, Lewis acid catalysis, and enzymatic systems—enabling asymmetric transformations of stabilized alkyl radicals. These radicals often benefit from conjugation effects or equipped with directing groups that facilitate the formation of stabilized high-valent metal intermediates. Nevertheless, the asymmetric manipulation of unactivated radicals, the most reactive subclass of these intermediates, has remained unconquered for decades. This long-standing challenge continues to drive intensive investigations among leading researchers globally, positioning its resolution as a paramount pursuit in modern chemistry.

Chair Professor Xin-Yuan Liu’s research group from the Department of Chemistry at the Southern University of Science and Technology (SUSTech), in collaboration with Professor Xin Hong’s team from Zhejiang University (ZJU), has recently achieved a breakthrough in enantioconvergent cross-coupling of dialkyl-substituted radicals.
Their paper, entitled “Asymmetric amination of alkyl radicals with two minimally different alkyl substituents”, has been published in Science.
To address this challenge, Professor Liu’s group has systematically pursued groundbreaking investigations since 2019, culminating in the development of a novel class of spatially confined chiral catalysts. These catalysts feature a truncated cone-shaped chiral pocket characterized by a sterically constrained region proximal to the catalytic center and an expanded spatial cavity at the periphery. During catalytic cycles, alkyl substituents with minimal steric demand preferentially occupy the confined interior zone of the catalytic pocket, while bulkier alkyl groups are sterically excluded to the peripheral spacious cavity.
Strategically positioned high-steric-demand moieties along the pocket periphery engage in multifaceted noncovalent weak interactions—such as van der Waals forces and C–H···π contacts—with the alkyl substrates. This synergistic design helps stabilizes the highly activated alkyl radical intermediates and significantly enhances stereodifferentiation capability between two minimally different alkyl groups. This stereocontrol model marks a pivotal advancement in catalyst architecture, establishing a versatile and generalizable strategy to address long-standing fundamental challenges in asymmetric catalysis. This work demonstrates remarkable potential for broad applicability across diverse catalytic systems, thereby redefining conventional approaches to stereoselective radical transformations.
The researchers successfully implemented this catalytic system in asymmetric amination of dialkyl iodoalkanes derived from industrial feedstocks, achieving efficient enantioselective amination of multiple substrate types. Notably, the system demonstrated accurately stereodifferentiation capability for substrates with two minimal alkyl groups, including methyl/propyl groups (2-iodopentane) and ethyl/propyl counterparts (3-iodohexane).
This methodology has enabled the first example of asymmetric C–H amination of unfunctionalized alkanes, thereby establishing a robust foundation for valorizing bulk industrial feedstocks into high-value chiral amine derivatives. Furthermore, the chiral primary amines synthesized via this protocol can be efficiently transformed into active pharmaceutical ingredients (APIs) or key intermediates of drugs, including the anticancer agent Ibrutinib and antidiabetic drugs Alogliptin and Trelagliptin. These findings provide crucial technological support for China’s long-term innovation in frontier pharmaceutical research.
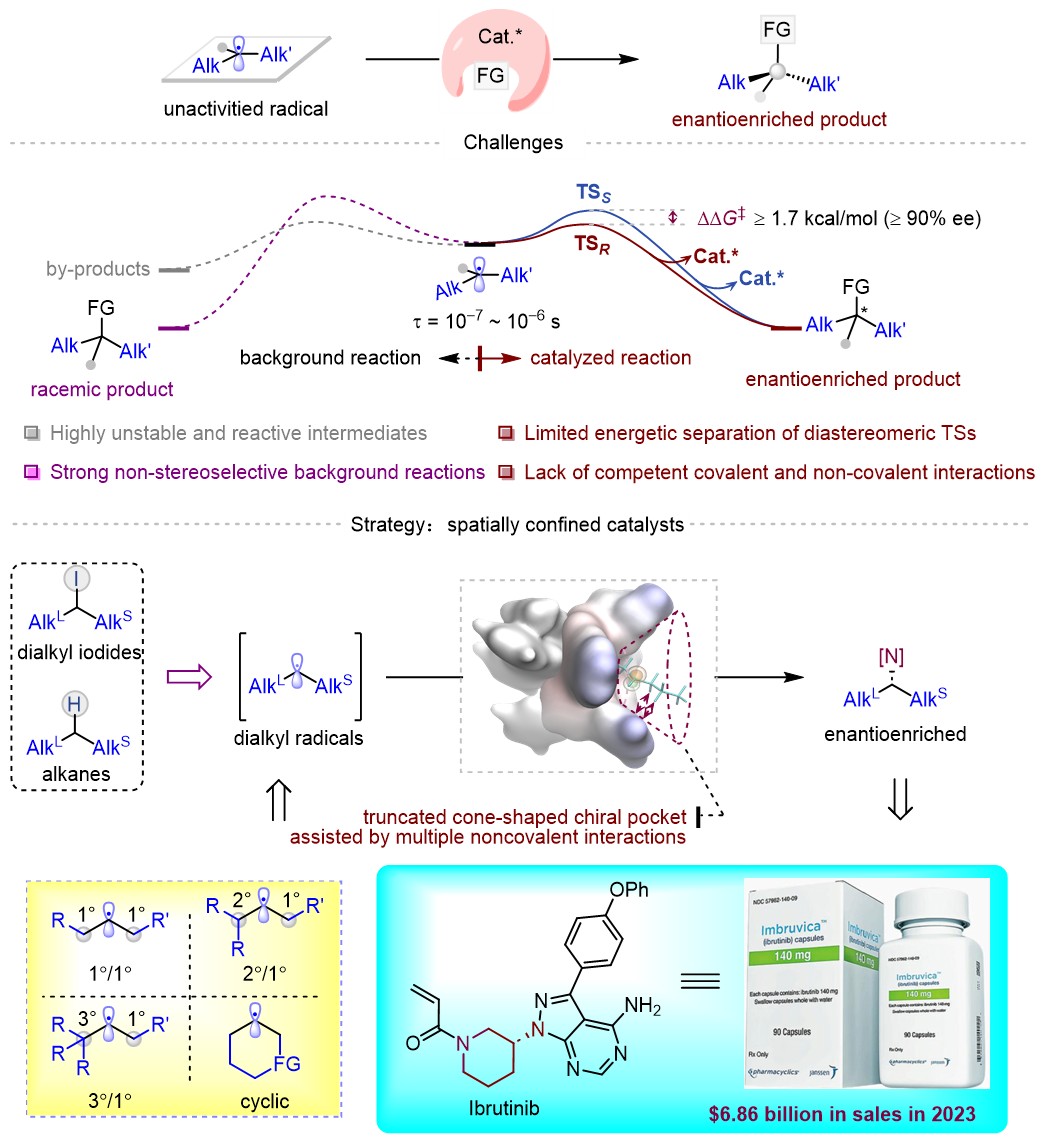
Figure 1. Asymmetric amination of alkyl radicals with two minimally different alkyl substituents
Research Associate Professor Yu-Feng Zhang from the Shenzhen Grubbs Institute and Guangming Advanced Research Institute at SUSTech is the first authors of the paper. Postdoctoral fellows Biao Wang and Ji-Ren Liu, along with master’s student Zheng Chen, all from SUSTech, are the co-first authors. Chair Professor Xin-Yuan Liu and Professor Xin Hong are the corresponding authors, with SUSTech serving as the first corresponding institution.
Other contributors to this study from SUSTech include Ning-Yuan Yang, Jin-Min Xiang, Juan Liu, and Qiang-Shuai Gu, Zhe Dong, Jianchun Wang, Cong Yu, Jiayuan Dong, Qiao Song, Yinhua Yang, and Lin Lin.
Paper link: www.science.org/doi/10.1126/science.adu3996




