Researchers pioneer asymmetric synthesis of nitrogen chirality San WU | 11/13/2025
2025-11-13
The exploration of N-chirality dates back to Werner’s initial proposal in 1890, yet stable chirality in trisubstituted amines was not confirmed until 1944 with the resolution of Tröger’s base, whose rigidity enabled configurational stability. Later, Brios et al. (1968) identified stable N-chirality in non-bridged N-chloroaziridines, while acyclic amines—due to lack of ring strain—exhibited pronounced instability. Further progress came in 1981, when Kostyanovsky’s team resolved an acyclic N-chiral compound with low enantiomer excess (ee), providing the first evidence of unstable N-chirality in flexible systems (Figure 1a).
Despite extensive efforts, progress in N-chirality has long been limited to configurational stability and chemical resolution. The low inversion barrier of acyclic N-stereocenters impeded direct asymmetric synthesis, restricting most advances to cyclic systems and often requiring stoichiometric chiral auxiliaries with poor selectivity (Figure 1b). Developing efficient catalytic asymmetric methods for acyclic N-chirality remains a major longstanding challenge.

A research team led by Chair Professor Bin Tan from the Department of Chemistry at the Southern University of Science and Technology (SUSTech), in collaboration with Professor Kendall N. Houk’s team at the University of California, Los Angeles (UCLA), has made a significant breakthrough in nitrogen chirality. For the first time, they have achieved the catalytic asymmetric synthesis of N-stereogenic compounds using organocatalysis, overcoming the inherent instability of nitrogen-centered chiral configurations.
Their findings, titled “Controlling pyramidal nitrogen chirality by asymmetric organocatalysis,” have been published in the journal Nature.
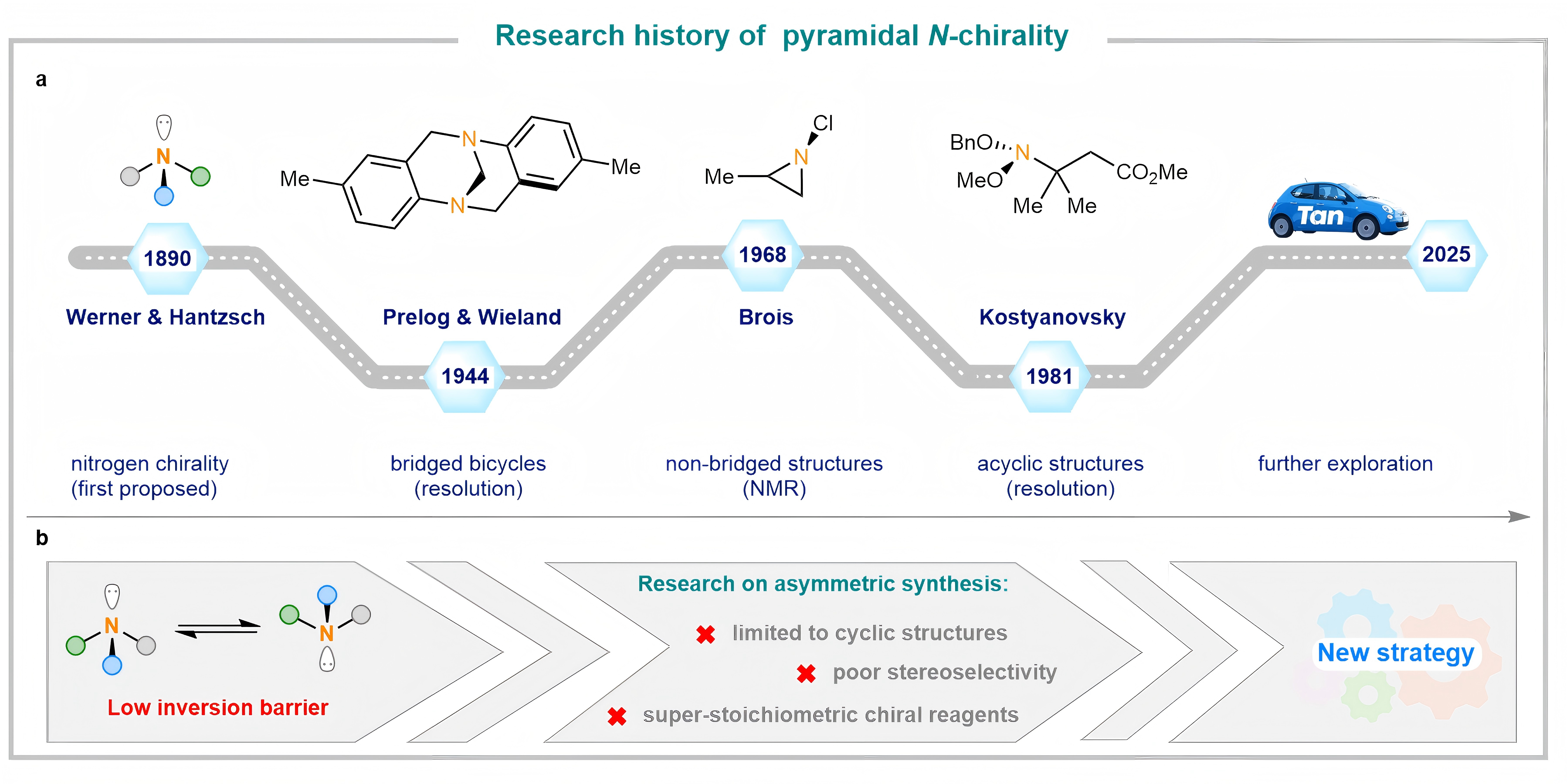
Figure 1. The research background of pyramidal N-chirality
Introducing strong electronegative substituents to the nitrogen center significantly raised the inversion barrier of N-chirality. For example, inversion barriers increased from 20–25 kJ/mol in aliphatic amines to 40–44 kJ/mol with alkoxy groups, 75–80 kJ/mol after chlorination, and 95–100 kJ/mol upon oxygen substitution (Figure 2a). The chlorination of hydroxylamines gradually converted achiral precursors to chiral molecules, offering a theoretical foundation for accessing acyclic N-chirogenic compounds via catalytic asymmetric chlorination (CAC). This approach was inspired by successful asymmetric halogenation strategies previously applied to carbon stereocenters.
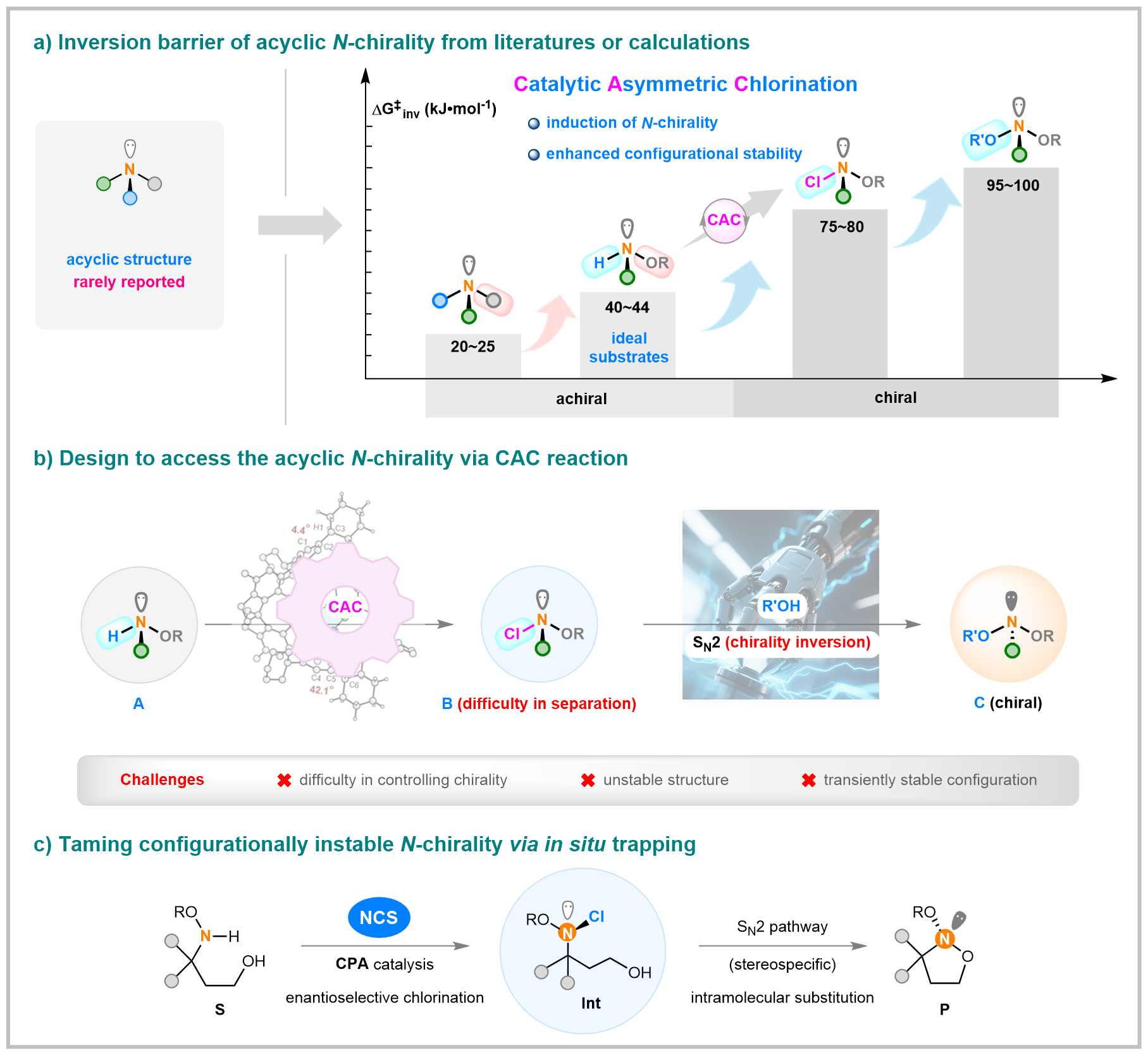
Figure 2. Rational design enables controlling the acyclic N-chirality
Guided by this principle, the researchers developed an asymmetric chlorination strategy to synthesize acyclic N-chirogenic chlorinated hydroxylamines. They then designed a nucleophilic substitution process to capture the unstable intermediate B, yielding configurationally stable N-chiral derivatives (Figure 2b). Effective chirality transfer required an SN2 mechanism with a rate far exceeding racemization. However, this approach faced three key challenges, including stereocontrol difficulties due to the nitrogen lone pair and low steric hindrance, intermediate instability leading to side reactions, and a low racemization barrier. Through systematic optimization, catalytic asymmetric chlorination was achieved, where pre-installed hydroxyl groups enabled accelerated intramolecular substitution with high fidelity, affording stable N-chirogenic products (Figure 2c).
The team employed a sterically hindered chiral phosphoric acid (CPA) as the catalyst. Through a two-step tandem process involving CAC reaction and intramolecular nucleophilic substitution, they successfully achieved highly enantioselective synthesis of N-stereogenic 1,2-oxazolidine compounds bearing nitrogen as the sole chiral element (Figure 3a). This strategy demonstrated broad substrate scope and excellent functional group compatibility. The structures and absolute configuration were unambiguously confirmed by single-crystal X-ray diffraction analysis, which clearly revealed a pyramidal geometry at the nitrogen center.
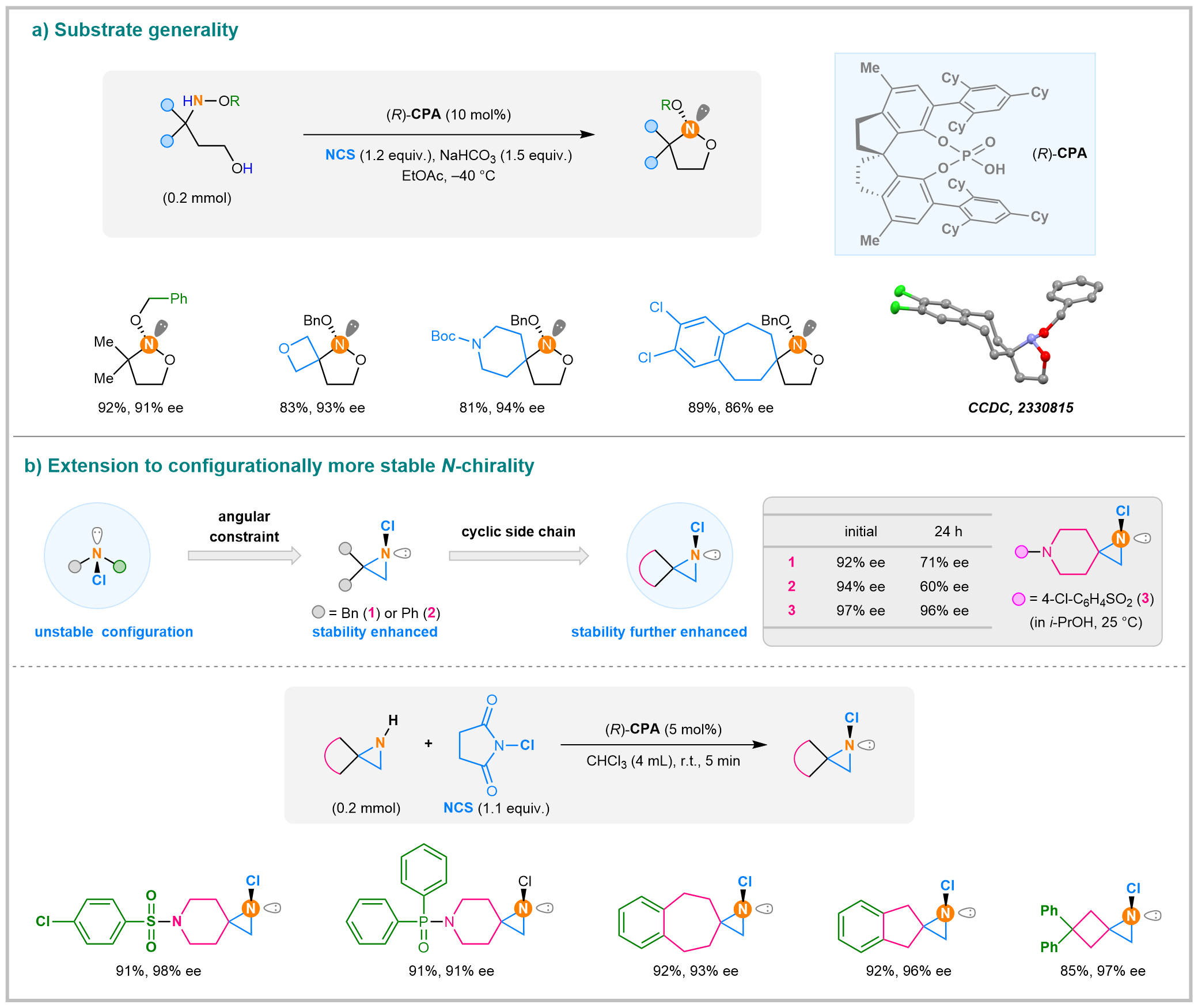
Figure 3. Organocatalytic asymmetric control of N-chirality
Although the ring strain in three-membered aziridines enhances the configurational stability of N-chloroaziridines compared to their acyclic analogues, significant racemization was still observed at room temperature for derivatives bearing adjacent dibenzyl (Figure 1) or diphenyl substituents (Figure 2). To address this issue, the team introduced a rigid spirocyclic structure adjacent to the nitrogen atom, which markedly improved configurational stability, showing only 1% ee loss under identical conditions (Figure 3b). Building on the success in catalytic asymmetric chlorination, they made slight adjustments to achieve highly enantioselective chlorination of aziridines. Using the same chiral phosphoric acid catalyst in chloroform, the reaction was completed within 5 minutes. The resulting products were obtained with ee values exceeding 90%.
To further probe the mechanism, they conducted in-depth mechanistic studies through control experiments and DFT theoretical calculations. These analyses confirmed that the stereoselectivity-determining step is the chlorination reaction, while the subsequent intramolecular nucleophilic substitution follows an SN2 mechanism, thereby ensuring efficient transfer of chiral information. This study represents the first catalytic asymmetric synthesis of N-chirogenic compounds and provides an important foundation and direction for subsequent research.
Research Assistant Professors San Wu and Pengquan Chen at SUSTech, along with postdoctoral fellow Meng Duan (theoretical calculations) at UCLA, are co-first authors of the paper. Chair Professor Bin Tan is the lead corresponding author, while Professor Kendall N. Houk is the co-corresponding author. SUSTech is the primary corresponding institution. Additional contributors to this work include postdoctoral fellow Pengying Jiang from the Department of Chemistry at SUSTech, Research Professor Shaohua Xiang from the Grubbs Institute at SUSTech, and Dr. Qingyang Zhou from UCLA.
Paper link: https://www.nature.com/articles/s41586-025-09607-6