Brain chemistry and neural development improved following new research
2020-02-06
With the increase of neurological diseases such as Alzheimers disease and schizophrenia, recent research in Shenzhen could help scientists around the world establish the causes of such diseases and potential treatment pathways in the future.
Department of Biology Associate Professor Wei Zhiyi has led his research group at Southern University of Science and Technology (SUSTech) to make crucial breakthroughs in our understanding of the regulatory mechanisms in neural development. Their findings were published on January 10 in Nature Communications, in an article titled, “Structural basis of liprin-α-promoted LAP-RPTP clustering for modulation of phosphatase activity.”
The paper revealed how LAR-RPTP, a receptor-type protein tyrosine phosphatase (RPTP) important for building neuronal connection, is regulated by an intracellular regulation in response to extracellular signals during neuron growth, shedding lights on understanding how our brain works.
Brain functions rely on numerous connections, named synapses, between neurons for transducing signals. The defective formation of synapses leads to severe mental disorders. Neurons grow long axons to their target cells guided by various extracellular cues to connect to each other. During synapse formation, synaptic proteins are assembled at the presynaptic and postsynaptic terminals to form membrane microdomains for neuronal signal transduction. Some synaptic proteins control these critical processes by protein-protein interactions as well as post-translational modifications.
Among these synaptic proteins, LAR-RPTP is a cell receptor protein that regulates the phosphotyrosine level of its many substrates. It plays an essential role in several processes of neuronal development, including axon growth and synapse formation. LAR-RPTP enzymatic activity is highly regulated in different developmental stages. Although the RPTP family members have been studied for decades, little is known about the regulatory mechanisms of LAR-RPTP. Previous studies suggest that extracellular ligands interact with LAR-RPTP and induce the cell surface redistribution of LAR-RPTP. However, there is a significant lack of knowledge about the impact of the redistribution of the phosphatase on its activity.
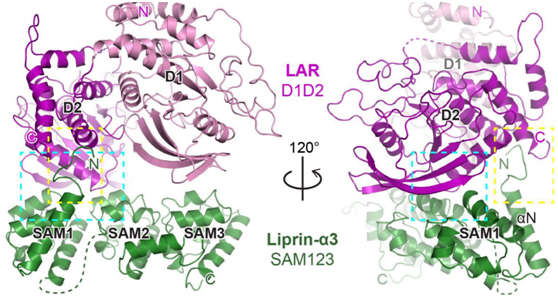
Figure 1:The overall structure of the liprin-α/LAR-RPTP complex
The research group combined biochemistry, structural biology and cell biology research methodology to investigate LAR-RPTP and its binding partner liprin-α. Extensive biochemical and crystallographic works drove the determination of the liprin-α/LAR-RPTP complex. The high-resolution structure clearly shows the binding mode of the two proteins, which was further confirmed by the mutagenesis study.
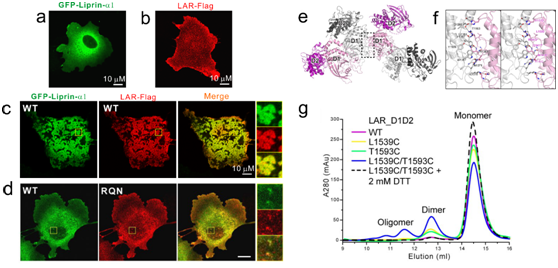
Figure 2: liprin-α promotes cluster formation of LAR in cells
Their further works using mammalian cells shows that liprin-α promotes the cluster formation of LAR-RPTP on the cell surface. The LAR-RPTP clustering depends on the interaction between liprin-α and LAR-RPTP as well as the oligomerization of liprin-α. These results demonstrated that liprin-α, as a vital scaffold in synaptogenesis, triggers the clustering of LAR-RPTP within the cell.
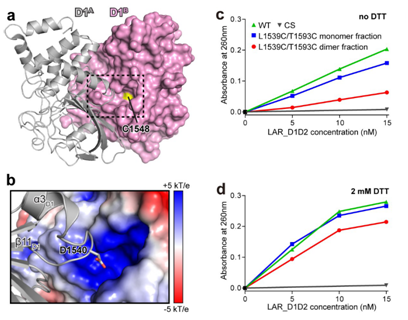
Figure 3: The clustering of LAR interferes with its phosphatase activity
The group’s analysis found the self-association of the phosphatase domain in LAR-RPTP. They proposed that LAR-RPTP forms clusters by their phosphatase domain, preventing its substrate from entering the active site for catalysis. They also determined that the liprin-α-promoted clustering attenuate the phosphatase activity of LAR-RPTP.
Since the phosphotyrosine level controls many cellular signaling, their discovery provides new insights for the regulation mechanism of cell behavior during neuronal development. This research lays the foundation for understanding the molecular basis of defective synapse formation in certain neurological diseases as well as finding strategies to cure similar diseases in the future.
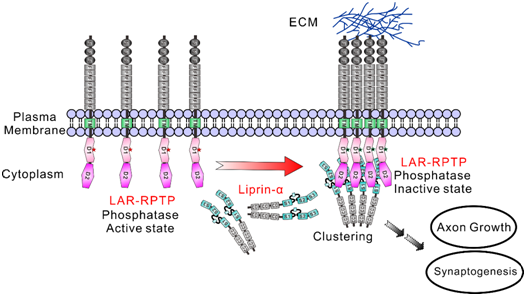
Figure 4: A proposed model of liprin-α-promoted LAR-RPTP clustering for modulation of phosphatase activity.
SUSTech Academy of Advanced Interdisciplinary Sciences (AAIS) Research Assistant Professor Xie Xingqiao was the first author, with research assistant Liang Mingfu & masters’ graduate Luo Ling making essential contributions to the paper. Associate Professor Wei Zhiyi is the sole correspondent author of the paper, with SUSTech as the first communication unit.
The research was strongly supported by the National Natural Science Foundation of China (NSFC), the Natural Science Foundation of Guangdong Province, the Science and Technology Planning Project of Guangdong Province, the Shenzhen-Hong Kong Institute of Brain Science, the Shenzhen Fundamental Research Institutions, and the Shenzhen Science and Technology Innovation Commission. The researchers thank the SUSTech Core Research Facilities and the Shanghai Synchrotron Radiation Facility for their assistance during data collection.
Article link: https://www.nature.com/articles/s41467-019-13949-x




