Solar cells to become more efficient with 3D interpenetrated acceptor, following SUSTech-led research
2020-04-13
Organic solar cells are becoming increasingly efficient, and new developments by researchers at Department of Chemistry, Southern University of Science and Technology (SUSTech) has seen a significant step forward in that area.
Associate Professor Feng He (Chemistry) has led his research group to make crucial breakthroughs in designing and synthesizing high-performance non-fullerene acceptors for the development of organic solar cells. Their findings were published on March 09 in the high-impact academic journal Joule (IF = X.XX), in an article titled, “Trifluoromethylation Enables a 3D Interpenetrated Low-Bandgap Acceptor for Efficient Organic Solar Cells”.
With the emergence of nonfullerene acceptors (NFAs) in recent years, the power conversion efficiencies (PCEs) of organic solar cells (OSCs) had broken through the bottleneck of 12% based on fullerene acceptors. The PCEs of single-junction OSCs were boosted to over 15% in a fast speed, and more than 16% of PCEs were realized in ternary OSCs. The rapid development of OSCs can be credited to the excellent flexibility of synthetic routes, absorption spectra, and energy levels of NFAs compared to fullerene acceptors.
One of the most significant advantages of NFAs was that the absorption would complement that of the polymer donors. It promotes sunlight harvesting and benefits the fabrication of transparent devices for commercial applications. Thence, designing low bandgap NFAs is considered to be a promising strategy to improve the performance of OSCs.
Trifluoromethylation has often been used to modify a variety of biological properties in bioactive compounds. However, they have rarely been applied to solar cells. The research team introduced the trifluoromethyl to electron-deficient malononitrile (INIC) end-capped moiety.
An ultra-narrow bandgap nonfullerene acceptor was obtained, benefiting from the strong electrostatic effect of trifluoromethyl groups. Three low bandgap NFAs were also synthesized with fluorine (F), chlorine (Cl), and trifluoromethyl end groups. In these four NFAs, BTIC-CF3–γ and BTIC-CF3–m demonstrated dramatically red-shifted absorption with peak values up to 838 nm.
It’s worth noting that BTIC-CF3–γ possessed the most red-shift absorption edge at 951 nm, corresponding to an ultra-narrow bandgap of 1.3 eV.
 Figure 1: The structures, optical properties, and energy levels of NFAs. (A) The chemical structures of four molecules. (B) Normalized UV-vis absorption of four NFAs in films. (C) The values of electrochemical energy levels for NFAs.
Figure 1: The structures, optical properties, and energy levels of NFAs. (A) The chemical structures of four molecules. (B) Normalized UV-vis absorption of four NFAs in films. (C) The values of electrochemical energy levels for NFAs.
 Figure 2: The single crystal structure of BTIC-CF3–γ. (A) Molecules are packing to elliptical frames that view from the x-y plane. (B) Molecules are packing to elliptical frames that view from the x-z plane. (C) Multiple intermolecular interactions, including F∙∙∙N, N∙∙∙S, F∙∙∙S, N∙∙∙N, and N∙∙∙S. (D) The top view of the planar network structure of BTIC-CF3–γ. (E) 3D interpenetrating network structure of BTIC-CF3–γ.
Figure 2: The single crystal structure of BTIC-CF3–γ. (A) Molecules are packing to elliptical frames that view from the x-y plane. (B) Molecules are packing to elliptical frames that view from the x-z plane. (C) Multiple intermolecular interactions, including F∙∙∙N, N∙∙∙S, F∙∙∙S, N∙∙∙N, and N∙∙∙S. (D) The top view of the planar network structure of BTIC-CF3–γ. (E) 3D interpenetrating network structure of BTIC-CF3–γ.
The 3D interpenetrating network should help change transportation in multiple directions. It is closer to the isotropy electron transportation features in fullerene acceptors for improved electron mobility in these acceptors.
After fabricated as OSCs devices, BTIC-CF3–m-based devices showed a PCE of 15.30%, which is about 12% and 16% enhancements in PCEs when compared with that of the fluorinated BTIC-F-mand chlorinated BTIC-Cl-m-based devices, respectively. The primary increase of PCE comes from a significant improvement in Jsc in BTIC-CF3–m acceptor, indicating that the trifluoromethylation was an effective strategy to improve photovoltaic properties compared to either single F or Cl substitutions in these kinds of NFAs. To further improve the performance of the devices, the strategy of isomer-free acceptor from the material side was executed by using pure BTIC-CF3–γ in the device fabrications. The PBDB-TF: BTIC-CF3-γ-based optimized devices exhibited PCEs of 15.59%. The PCE of 15.59% is the highest values in reported ultra-narrow bandgap NFAs to date. Moreover, the PCE of 16.50% by Y6: BTIC-CF3-γ (10: 1.15) ternary system indicates the potential values of BTIC-CF3-γ due to its red-shifted absorption.
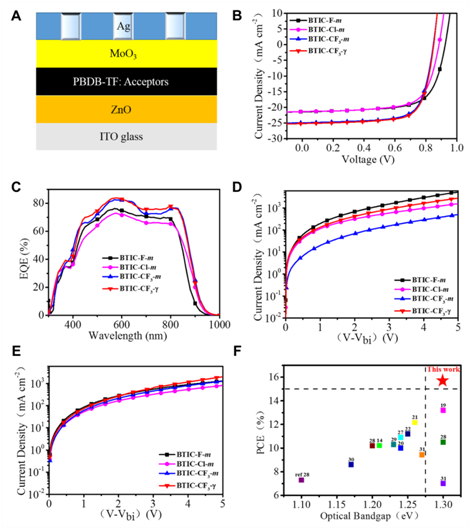 Figure 3: Photoelectric properties of NFA-based devices. (A) The structure of reversal OSCs devices. (B) The J–V curves of four NFAs-based devices. (C) The EQE curves of four NFAs-based devices. (D) The Electron mobility curves of four NFAs-based devices. (E) The hole mobility curves of four NFAs-based devices. (F) The PCEs of ultra-narrow bandgap NFAs reported to data.
Figure 3: Photoelectric properties of NFA-based devices. (A) The structure of reversal OSCs devices. (B) The J–V curves of four NFAs-based devices. (C) The EQE curves of four NFAs-based devices. (D) The Electron mobility curves of four NFAs-based devices. (E) The hole mobility curves of four NFAs-based devices. (F) The PCEs of ultra-narrow bandgap NFAs reported to data.
In conclusion, trifluoromethyl has been used to non-fullerene acceptors resulting in an ultra-narrow bandgap molecule, named BTIC-CF3-γ. Compared to fluorinated and chlorinated analogs, BTIC-CF3-γ possessed the most red-shifted absorption. This is the first report of the Y6 family single crystal. After PCEs of 15.59% is realized, it is presented here as the highest value in the reported ultra-narrow bandgap NFAs. Notably, A PCE of 16.5% is realized in the ternary system, which demonstrates the potential application values of BTIC-CF3-γ in ternary devices for its red-shifted absorptions. He’s group provides a visualized understanding of molecule packing by single-crystal analysis, and also suggest that the trifluoromethylation is a useful strategy for designing highly efficient acceptors with deep red-shifted sunlight absorptions.
Doctoral candidate Hanjian Lai is the first author of this paper. Associate Professor Feng He is the sole correspondent author, with SUSTech as the first unit and the sole communication unit. Additional contributions came from South China University of Technology.
This work was financially supported by the National Natural Science Foundation of China, Shenzhen Fundamental Research Program , Shenzhen Nobel Prize Scientists Laboratory Project, and Guangdong Innovative and Entrepreneurial Research Team Program. We also thank the SUSTech Core Research Facilities for the AFM and TEM measurements for this work.
Article link: https://www.sciencedirect.com/science/article/pii/S2542435120300829?via=ihub




