Prof. Julius Rebek Jr.: Recognition and Reactivity in Deep Cavitands
Pei WANG 2020-10-28
On October 27th, 2020, Prof. Julius Rebek Jr. from the Scripps Research Institute was invited to the 38th Science Lecture of College of Science. He gave a talk on “Recognition and Reactivity in Deep Cavitands”, which was chaired by Prof. Wei JIANG, Dept. of Chemistry. The lecture was held online via Zoom meeting.
Guest Introduction

Prof. Julius Rebek Jr. was born in Hungary in 1944. He completed his undergraduate study at the University of Kansas in 1966, and received his Ph.D degree from the Massachusetts Institute of Technology (MIT) in 1970. He worked as an Assistant Professor at the University of California at Los Angeles (1970-1976), as a professor at the University of Pittsburgh (1976-1989) and as the Camille Dreyfus Professor of Chemistry at MIT (1989-1996). Since 1996, Prof. Rebek has been working as a professor in the Scripps Research Institute and as the Director of the Skaggs Institute for Chemical Biology. Prof. Rebek is the member of National Academy of Science, American Academy of Arts and Sciences, European Academy of Science, as well as Royal Swedish Academy of Sciences. He has received numerous honors and awards, including ACS Distinguished Scientist Award, Chemical Pioneer Award, Humboldt Senior Scientist Award, Nichols Medal, Prelog Medal and so on. He is also the founder of several biopharmaceutical companies, including the public-traded company-Cubist Pharmaceuticals, which was purchased in 2014 by Merck for $9.5 million. Professor Julius Rebek Jr.’s study focuses on molecular recognition and catalysis, molecular self-assembly and encapsulation, sensors and antidotes for chemical warfare agents and protein surface mimetics.
Lecture Review
Molecular recognition is the basis of life and the central topic of supramolecular chemistry. In this report, Prof. Rebek introduced a series of water-soluble deep cavitands with different functional sidewalls. These synthetic receptors have good-to-excellent binding affinities to biorelevant molecular targets (for example, acetylcholine and choline) and hydrocarbons. The linear hydrocarbons tend to be bound and folded in the cavity, and adopt unusual conformations. On this basis, they further used their system to control the reactivity of certain molecules, achieving cyclization and monofucntionalization which would not be easily realized otherwise.
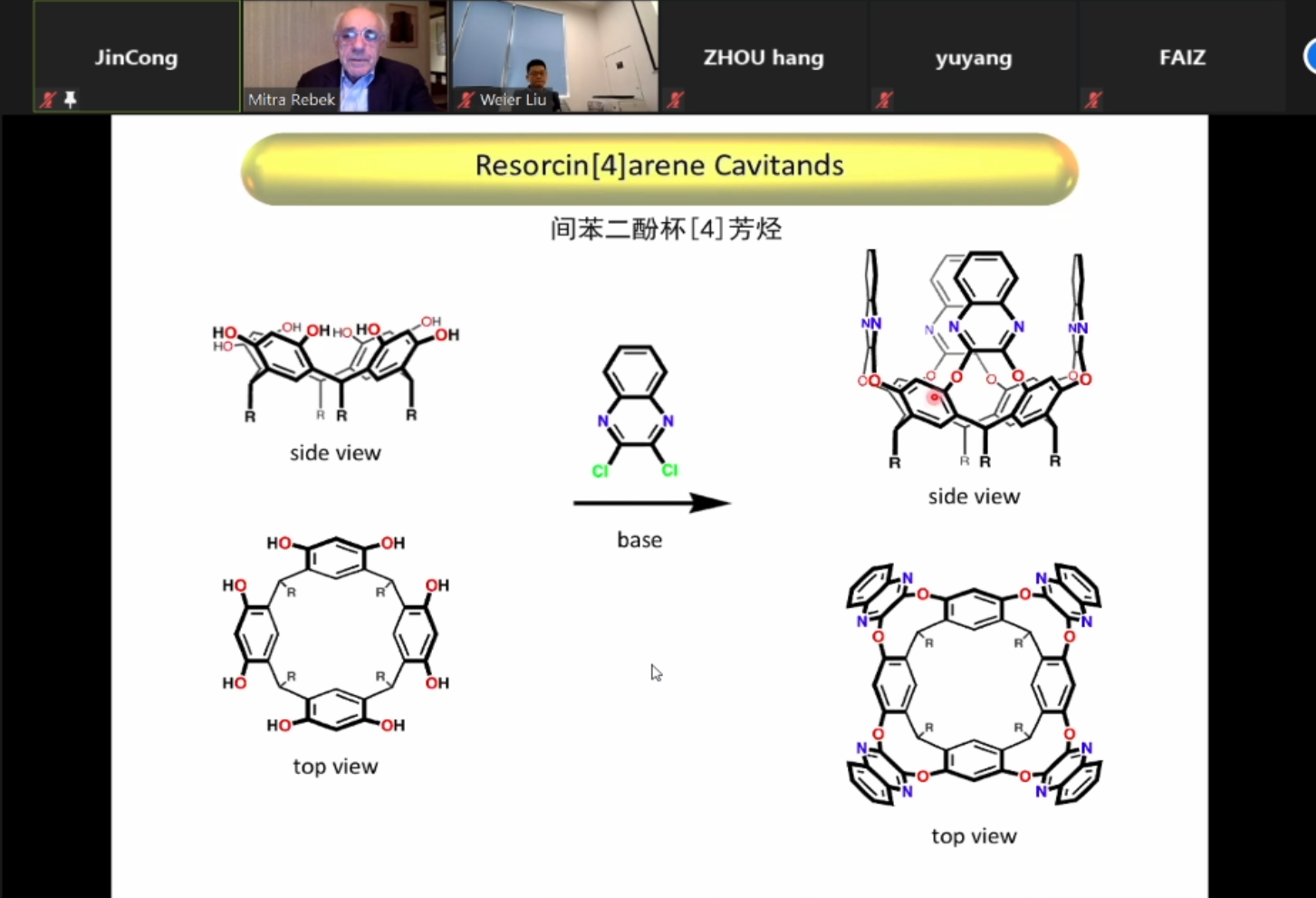
During the lecture, Prof. Rebek introduced the synthesis, conformations and dynamic properties of Resorcin[4]arene Cavitands, which mainly adopt two conformations -- vase and kite, and the two conformations can interconvert. To access a deeper and more stable cavitand, they introduced amide groups in the upward rim. The cavitand conformation is thus fixed as a vase conformation through the hydrogen bonds between the neighbouring amide groups. In the“molecular recognition" part, Prof. Rebek presented NMR spectra and X-ray crystal structures to support the adamantane binding in the cavitand. In the "Water-Soluble Cavitands" part, he shows the recognition ability to guests by using different cavitands under different pH values in water, which is driven by electrostatic interactions and hydrophobic effects. He further proposed the concept of Cavitand Domains to explain the phenomenon of guests folding. Especially for an octamethyl cavitand, when it binds guests such as alcohols, they found that with the length of chains increasing, the chain of guests would extend, bend and fold to U-shape, which was characterized by NMR.
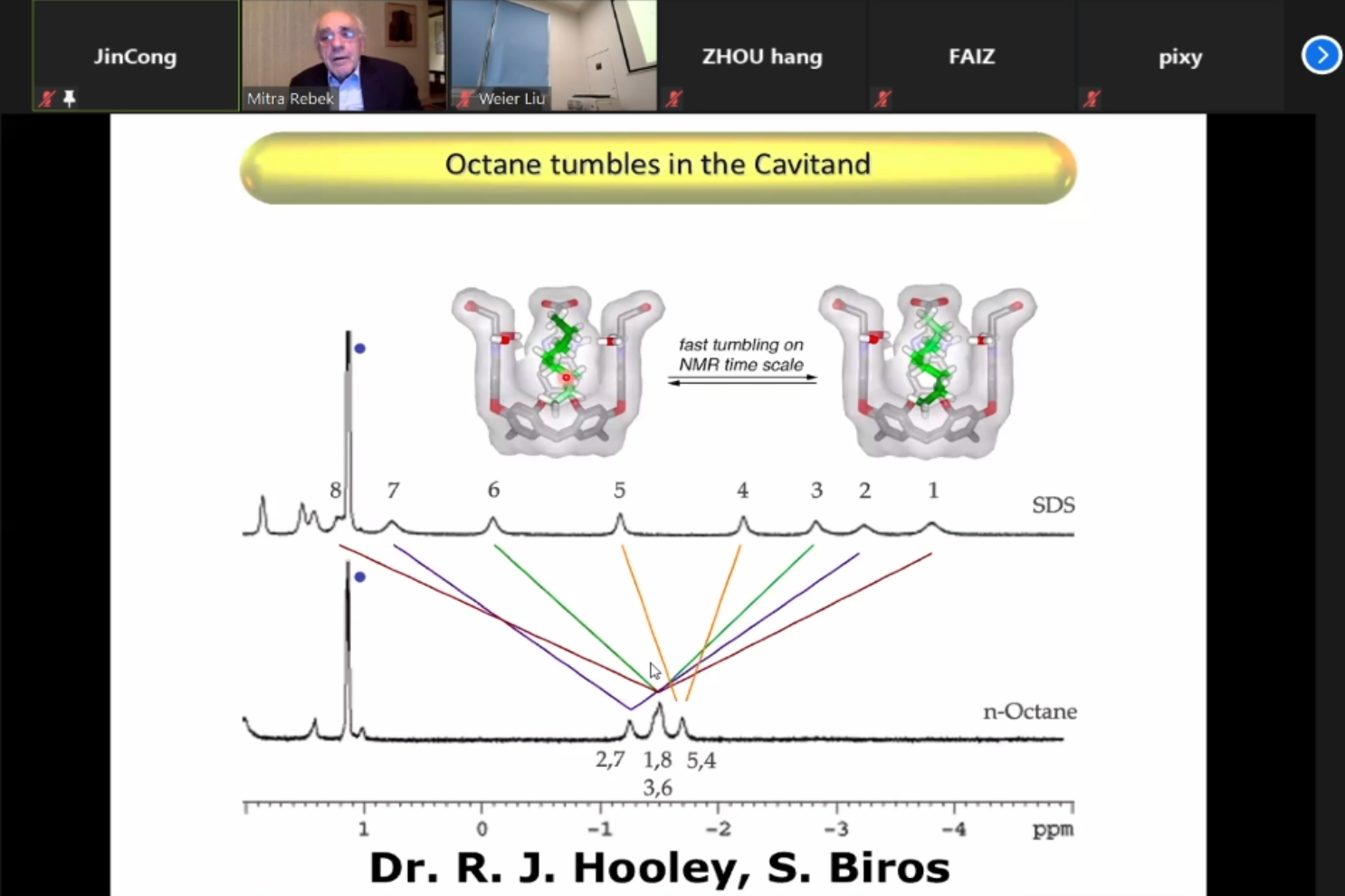
On this basis, they further studied the cyclization of the C12 amino acid in D2O by using the cavitand as a template. Besides, when the hydrocarbon derivatives are complexedin the cavitand with a wide opening, it adopts an interesting dynamic yo yo motion. One of the hydrocarbon ends is shielded from the outside, and thus monofunctionalization was achieved. The reactions include the hydrolysis of esters and Staudinger Reaction of Cn Diazides. In the next part, Prof. Rebek introduced several new water-soluble cavitands such as metallo cavitand, which shows high selectivity to p-xylene and potentials in industrial separation. In the last part, Prof. Rebek showed even deeper water-soluble cavitands, which are based on pre-stacked aromatics. They further studied their binding to fatty acids in 5% DMSO in D2O. From this model, it's clear that the methyl group is deep inside the cavitand, while the hydrophilic tail is outside and in the solvent.
Interaction
There are 70 audience who attended the lecture online and 10 who attended the lecture at the conference room. After the lecture, the audience asked several questions to Prof. Julius Rebek regarding the crystal structures of the cavitands, the contribution of CH...π interactions in binding alkanes, biological applications of the cavitands and so on. Prof. Rebek gave detailed explanations to each question.

Students listening to the lecture




